The reaper-binding protein scythe modulates apoptosis and proliferation during mammalian development
- PMID: 16287848
- PMCID: PMC1291232
- DOI: 10.1128/MCB.25.23.10329-10337.2005
The reaper-binding protein scythe modulates apoptosis and proliferation during mammalian development
Abstract
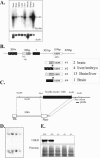
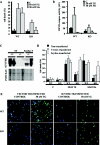
Scythe (BAT3 [HLA-B-associated transcript 3]) is a nuclear protein that has been implicated in apoptosis, as it can modulate Reaper, a central apoptotic regulator in Drosophila melanogaster. While Scythe can markedly affect Reaper-dependent apoptosis in Xenopus laevis cell extracts, the function of Scythe in mammals is unknown. Here, we report that inactivation of Scythe in the mouse results in lethality associated with pronounced developmental defects in the lung, kidney, and brain. In all cases, these developmental defects were associated with dysregulation of apoptosis and cellular proliferation. Scythe-/- cells were also more resistant to apoptosis induced by menadione and thapsigargin. These data show that Scythe is critical for viability and normal development, probably via regulation of programmed cell death and cellular proliferation.
Figures





Similar articles
-
Scythe regulates apoptosis-inducing factor stability during endoplasmic reticulum stress-induced apoptosis.J Biol Chem. 2008 Feb 8;283(6):3264-3271. doi: 10.1074/jbc.M706419200. Epub 2007 Dec 3. J Biol Chem. 2008. PMID: 18056262
-
Human Scythe contains a functional nuclear localization sequence and remains in the nucleus during staurosporine-induced apoptosis.Biochem Biophys Res Commun. 2001 Oct 12;287(5):1075-82. doi: 10.1006/bbrc.2001.5701. Biochem Biophys Res Commun. 2001. PMID: 11587531
-
Scythe: a novel reaper-binding apoptotic regulator.EMBO J. 1998 Nov 2;17(21):6135-43. doi: 10.1093/emboj/17.21.6135. EMBO J. 1998. PMID: 9799223 Free PMC article.
-
Apoptosis in preimplantation mammalian embryo and genetics.Ital J Anat Embryol. 2001;106(2 Suppl 2):101-8. Ital J Anat Embryol. 2001. PMID: 11732565 Review.
-
Profiling gene expression in growth-arrested mouse embryos in diapause.Genome Biol. 2005;6(1):202. doi: 10.1186/gb-2004-6-1-202. Epub 2004 Dec 21. Genome Biol. 2005. PMID: 15642106 Free PMC article. Review.
Cited by
-
E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates.Infect Immun. 2010 Sep;78(9):3905-19. doi: 10.1128/IAI.00344-10. Epub 2010 Jun 14. Infect Immun. 2010. PMID: 20547746 Free PMC article.
-
An essential role of intestinal cell kinase in lung development is linked to the perinatal lethality of human ECO syndrome.FEBS Lett. 2017 May;591(9):1247-1257. doi: 10.1002/1873-3468.12644. Epub 2017 Apr 21. FEBS Lett. 2017. PMID: 28380258 Free PMC article.
-
The Chaperone BAG6 Regulates Cellular Homeostasis between Autophagy and Apoptosis by Holding LC3B.iScience. 2020 Oct 21;23(11):101708. doi: 10.1016/j.isci.2020.101708. eCollection 2020 Nov 20. iScience. 2020. PMID: 33241194 Free PMC article.
-
The roles of cytosolic quality control proteins, SGTA and the BAG6 complex, in disease.Adv Protein Chem Struct Biol. 2019;114:265-313. doi: 10.1016/bs.apcsb.2018.11.002. Epub 2018 Dec 18. Adv Protein Chem Struct Biol. 2019. PMID: 30635083 Free PMC article. Review.
-
BAT3 interacts with transforming growth factor-beta (TGF-beta) receptors and enhances TGF-beta1-induced type I collagen expression in mesangial cells.J Biol Chem. 2008 Jul 11;283(28):19816-25. doi: 10.1074/jbc.M802285200. Epub 2008 May 16. J Biol Chem. 2008. PMID: 18487607 Free PMC article.
References
-
- Ball, E. M., and G. P. Risbridger. 2001. Activins as regulators of branching morphogenesis. Dev. Biol. 238:1-12. - PubMed
-
- Bouchard, M. 2004. Transcriptional control of kidney development. Differentiation 72:295-306. - PubMed
-
- Brodbeck, S., and C. Englert. 2004. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr. Nephrol. 19:249-255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
