Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation
- PMID: 16287860
- PMCID: PMC1291235
- DOI: 10.1128/MCB.25.23.10479-10491.2005
Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation
Abstract
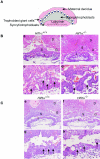
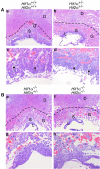
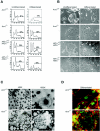
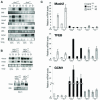
Placental development initially occurs in a low-oxygen (O2) or hypoxic environment. In this report we show that two hypoxia-inducible factors (HIFs), HIF1alpha and HIF2alpha, are essential for determining murine placental cell fates. HIF is a heterodimer composed of HIFalpha and HIFbeta (ARNT) subunits. Placentas from Arnt-/- and Hif1alpha-/- Hif2alpha-/- embryos exhibit defective placental vascularization and aberrant cell fate adoption. HIF regulation of Mash2 promotes spongiotrophoblast differentiation, a prerequisite for trophoblast giant cell differentiation. In the absence of Arnt or Hifalpha, trophoblast stem cells fail to generate these cell types and become labyrinthine trophoblasts instead. Therefore, HIF mediates placental morphogenesis, angiogenesis, and cell fate decisions, demonstrating that O2 tension is a critical regulator of trophoblast lineage determination. This novel genetic approach provides new insights into the role of O2 tension in the development of life-threatening pregnancy-related diseases such as preeclampsia.
Figures



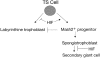
Similar articles
-
Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta.Development. 2005 Aug;132(15):3393-403. doi: 10.1242/dev.01923. Epub 2005 Jun 29. Development. 2005. PMID: 15987772
-
Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses.Genes Dev. 2000 Dec 15;14(24):3191-203. doi: 10.1101/gad.853700. Genes Dev. 2000. PMID: 11124810 Free PMC article.
-
Hypoxia, HIF and the placenta.Cell Cycle. 2006 Mar;5(5):495-8. doi: 10.4161/cc.5.5.2497. Epub 2006 Mar 1. Cell Cycle. 2006. PMID: 16552177
-
Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development.Placenta. 2010 Nov;31(11):951-7. doi: 10.1016/j.placenta.2010.08.008. Epub 2010 Sep 24. Placenta. 2010. PMID: 20869770 Review.
-
Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy.Hum Reprod Update. 2010 Jul-Aug;16(4):415-31. doi: 10.1093/humupd/dmp046. Epub 2009 Nov 19. Hum Reprod Update. 2010. PMID: 19926662 Free PMC article. Review.
Cited by
-
Low chorionic villous succinate accumulation associates with recurrent spontaneous abortion risk.Nat Commun. 2021 Jun 8;12(1):3428. doi: 10.1038/s41467-021-23827-0. Nat Commun. 2021. PMID: 34103526 Free PMC article.
-
Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice.Mol Cell Biol. 2007 Jun;27(11):4142-56. doi: 10.1128/MCB.01932-06. Epub 2007 Mar 19. Mol Cell Biol. 2007. PMID: 17371845 Free PMC article.
-
Targeted Disruption of the MORG1 Gene in Mice Causes Embryonic Resorption in Early Phase of Development.Biomolecules. 2023 Jun 24;13(7):1037. doi: 10.3390/biom13071037. Biomolecules. 2023. PMID: 37509073 Free PMC article.
-
Transcriptional regulators of the trophoblast lineage in mammals with hemochorial placentation.Reproduction. 2014 Dec;148(6):R121-36. doi: 10.1530/REP-14-0072. Epub 2014 Sep 4. Reproduction. 2014. PMID: 25190503 Free PMC article. Review.
-
Benzo(a)pyrene and Cerium Dioxide Nanoparticles in Co-Exposure Impair Human Trophoblast Cell Stress Signaling.Int J Mol Sci. 2023 Mar 12;24(6):5439. doi: 10.3390/ijms24065439. Int J Mol Sci. 2023. PMID: 36982514 Free PMC article.
References
-
- Abbott, B. D., and M. R. Probst. 1995. Developmental expression of two members of a new class of transcription factors. II. Expression of aryl hydrocarbon receptor nuclear translocator in the C57BL/6N mouse embryo. Dev. Dyn. 204:144-155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
