The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans
- PMID: 16287872
- PMCID: PMC1291223
- DOI: 10.1128/MCB.25.23.10611-10627.2005
The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans
Abstract
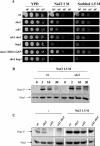
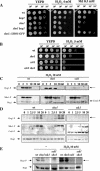
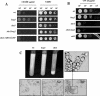
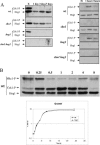
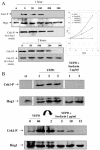
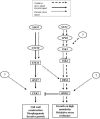
The Sho1 adaptor protein is an important element of one of the two upstream branches of the high-osmolarity glycerol (HOG) mitogen-activated protein (MAP) kinase pathway in Saccharomyces cerevisiae, a signal transduction cascade involved in adaptation to stress. In the present work, we describe its role in the pathogenic yeast Candida albicans by the construction of mutants altered in this gene. We report here that sho1 mutants are sensitive to oxidative stress but that Sho1 has a minor role in the transmission of the phosphorylation signal to the Hog1 MAP kinase in response to oxidative stress, which mainly occurs through a putative Sln1-Ssk1 branch of the HOG pathway. Genetic analysis revealed that double ssk1 sho1 mutants were still able to grow on high-osmolarity media and activate Hog1 in response to this stress, indicating the existence of alternative inputs of the pathway. We also demonstrate that the Cek1 MAP kinase is constitutively active in hog1 and ssk1 mutants, a phenotypic trait that correlates with their resistance to the cell wall inhibitor Congo red, and that Sho1 is essential for the activation of the Cek1 MAP kinase under different conditions that require active cell growth and/or cell wall remodeling, such as the resumption of growth upon exit from the stationary phase. sho1 mutants are also sensitive to certain cell wall interfering compounds (Congo red, calcofluor white), presenting an altered cell wall structure (as shown by the ability to aggregate), and are defective in morphogenesis on different media, such as SLAD and Spider, that stimulate hyphal growth. These results reveal a role for the Sho1 protein in linking oxidative stress, cell wall biogenesis, and morphogenesis in this important human fungal pathogen.
Figures




References
-
- Arana, D. M., C. Nombela, R. Alonso-Monge, and J. Pla. 2005. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 151:1033-1049. - PubMed
-
- Ausubel, F. M., et al. (ed.). 1993. Current protocols in molecular biology. Greene Publishing Associates, New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
