Unique structure of Ascaris suum b5-type cytochrome: an additional alpha-helix and positively charged residues on the surface domain interact with redox partners
- PMID: 16288599
- PMCID: PMC1408674
- DOI: 10.1042/BJ20051308
Unique structure of Ascaris suum b5-type cytochrome: an additional alpha-helix and positively charged residues on the surface domain interact with redox partners
Abstract
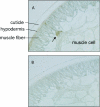
Cytochrome b5 of the body wall of adult Ascaris suum, a porcine parasitic nematode, is a soluble protein that lacks a C-terminal membrane-anchoring domain, but possesses an N-terminal pre-sequence of 30 amino acids. During the maturation of cytochrome b5, the N-terminal pre-sequence is proteolytically cleaved to form the mature protein of 82 amino acid residues. A. suum cytochrome b5 is a basic protein containing more lysine residues and exhibiting a higher midpoint redox potential than its mammalian counterparts. We developed an expression system for the production of the recombinant nematode cytochrome b5, which is chemically and functionally identical with the native protein. Using this recombinant protein, we have determined the X-ray crystal structure of A. suum cytochrome b5 at 1.8 A (1 A=0.1 nm) resolution, and we have shown that this protein is involved in the reduction of nematode body-wall metmyoglobin. The crystal structure of A. suum cytochrome b5 consists of six alpha-helices and five beta-strands. It differs from its mammalian counterparts by having a head-to-tail disulphide bridge, as well as a four-residue insertion in the vicinity of the sixth ligating histidine, which forms an additional alpha-helix, alpha4A, between helices alpha4 and alpha5. A. suum cytochrome b5 exists predominantly as a haem-orientation B isomer. Furthermore, the haem plane is rotated approx. 80 degrees relative to the axis formed by haem-Fe and N atoms of the two histidine residues that are ligated to haem-Fe. The charge distribution around the haem crevice of A. suum cytochrome b5 is remarkably different from that of mammalian cytochrome b5 in that the nematode protein bears positively charged lysine residues surrounding the haem crevice. Using immunohistochemistry, we found that A. suum cytochrome b5 is present in the nematode hypodermis. Based on this histochemical and structural information, the physiological function of A. suum cytochrome b5 and its interaction with nematode metmyoglobin can be hypothesized.
Figures



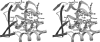




Similar articles
-
Bioinformatic identification of cytochrome b5 homologues from the parasitic nematode Ascaris suum and the free-living nematode Caenorhabditis elegans highlights the crucial role of A. suum adult-specific secretory cytochrome b₅ in parasitic adaptation.Parasitol Int. 2016 Apr;65(2):113-20. doi: 10.1016/j.parint.2015.11.004. Epub 2015 Nov 10. Parasitol Int. 2016. PMID: 26571414
-
Heterologous expression of Ascaris suum cytochrome b5 precursor protein: a histidine-tagged full-length presequence is correctly processed to transport the mature protein to the periplasm of Escherichia coli.Arch Biochem Biophys. 2003 May 15;413(2):253-61. doi: 10.1016/s0003-9861(03)00124-3. Arch Biochem Biophys. 2003. PMID: 12729624
-
Purification and molecular characterization of a novel b5-type cytochrome of the parasitic nematode, Ascaris suum.Arch Biochem Biophys. 1996 Apr 1;328(1):165-72. doi: 10.1006/abbi.1996.0157. Arch Biochem Biophys. 1996. PMID: 8638926
-
The cytochrome b5-fold: an adaptable module.Biochimie. 1994;76(7):674-92. doi: 10.1016/0300-9084(94)90144-9. Biochimie. 1994. PMID: 7893819 Review.
-
Electron transfer from cytochrome b5 to cytochrome c.J Bioenerg Biomembr. 1995 Jun;27(3):331-40. doi: 10.1007/BF02110102. J Bioenerg Biomembr. 1995. PMID: 8847346 Review.
Cited by
-
Structural and thermodynamic consequences of b heme binding for monomeric apoglobins and other apoproteins.Gene. 2007 Aug 15;398(1-2):12-28. doi: 10.1016/j.gene.2007.02.046. Epub 2007 May 1. Gene. 2007. PMID: 17550789 Free PMC article. Review.
References
-
- Lederer F. The cytochrome b5-fold: an adaptable module. Biochimie. 1994;76:674–692. - PubMed
-
- Vergeres G., Waskell L. Cytochrome b5, its functions, structure and membrane topology. Biochimie. 1995;77:604–620. - PubMed
-
- Kuroda R., Ikenoue T., Honsho M., Tsujimoto S., Mitoma J., Ito A. Charged amino acids at the carboxyl-terminal portions determine the intracellular locations of two isoforms of cytochrome b5. J. Biol. Chem. 1998;273:31097–31102. - PubMed
-
- Oshino N., Omura T. Immunochemical evidence for the participation of cytochrome b5 in microsomal stearyl-CoA desaturation reaction. Arch. Biochem. Biophys. 1973;157:395–404. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

