Factors influencing in vitro infectivity and growth of Rickettsia peacockii (Rickettsiales: Rickettsiaceae), an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni (Acari, Ixodidae)
- PMID: 16288906
- PMCID: PMC1625098
- DOI: 10.1016/j.jip.2005.09.001
Factors influencing in vitro infectivity and growth of Rickettsia peacockii (Rickettsiales: Rickettsiaceae), an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni (Acari, Ixodidae)
Abstract
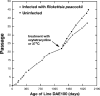
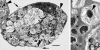
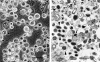
Rickettsia peacockii, a spotted fever group rickettsia, is a transovarially transmitted endosymbiont of Rocky Mountain wood ticks, Dermacentor andersoni. This rickettsia, formerly known as the East Side Agent and restricted to female ticks, was detected in a chronically infected embryonic cell line, DAE100, from D. andersoni. We examined infectivity, ability to induce cytopathic effect (CPE) and host cell specificity of R. peacockii using cultured arthropod and mammalian cells. Aposymbiotic DAE100 cells were obtained using oxytetracycline or incubation at 37 degrees C. Uninfected DAE100 sublines grew faster than the parent line, indicating R. peacockii regulation of host cell growth. Nevertheless, DAE100 cellular defenses exerted partial control over R. peacockii growth. Rickettsiae existed free in the cytosol of DAE100 cells or within autophagolysosomes. Exocytosed rickettsiae accumulated in the medium and were occasionally contained within host membranes. R. peacockii multiplied in other cell lines from the hard ticks D. andersoni, Dermacentor albipictus, Ixodes scapularis, and Ixodes ricinus; the soft tick Carios capensis; and the lepidopteran Trichoplusia ni. Lines from the tick Amblyomma americanum, the mosquito Aedes albopictus, and two mammalian cell lines were non-permissive to R. peacockii. High cell densities facilitated rickettsial spread within permissive cell cultures, and an inoculum of one infected to nine uninfected cells resulted in the greatest yield of infected tick cells. Cell-free R. peacockii also were infectious for tick cells and centrifugation onto cell layers enhanced infectivity approximately 100-fold. The ability of R. peacockii to cause mild CPE suggests that its pathogenicity is not completely muted. An analysis of R. peacockii-cell interactions in comparison to pathogenic rickettsiae will provide insights into host cell colonization mechanisms.
Figures

Similar articles
-
Sequence and expression analysis of the ompA gene of Rickettsia peacockii, an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni.Appl Environ Microbiol. 2004 Nov;70(11):6628-36. doi: 10.1128/AEM.70.11.6628-6636.2004. Appl Environ Microbiol. 2004. PMID: 15528527 Free PMC article.
-
Isolation of a spotted fever group Rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line.Appl Environ Microbiol. 2001 Feb;67(2):546-52. doi: 10.1128/AEM.67.2.546-552.2001. Appl Environ Microbiol. 2001. PMID: 11157215 Free PMC article.
-
Phagocytosis of the Lyme disease spirochete, Borrelia burgdorferi, by cells from the ticks, Ixodes scapularis and Dermacentor andersoni, infected with an endosymbiont, Rickettsia peacockii.J Insect Sci. 2007;7:58. doi: 10.1673/031.007.5801. J Insect Sci. 2007. PMID: 20331397 Free PMC article.
-
Recent advances in understanding tick and rickettsiae interactions.Parasite Immunol. 2021 May;43(5):e12830. doi: 10.1111/pim.12830. Epub 2021 Apr 15. Parasite Immunol. 2021. PMID: 33713348 Free PMC article. Review.
-
[Emerging rickettsioses].Parassitologia. 2004 Jun;46(1-2):123-6. Parassitologia. 2004. PMID: 15305700 Review. Italian.
Cited by
-
Susceptibility of inbred mice to Rickettsia parkeri.Infect Immun. 2012 May;80(5):1846-52. doi: 10.1128/IAI.00109-12. Epub 2012 Mar 5. Infect Immun. 2012. PMID: 22392926 Free PMC article.
-
Rickettsial ompB promoter regulated expression of GFPuv in transformed Rickettsia montanensis.PLoS One. 2010 Jan 29;5(1):e8965. doi: 10.1371/journal.pone.0008965. PLoS One. 2010. PMID: 20126457 Free PMC article.
-
The Tick Cell Biobank: A global resource for in vitro research on ticks, other arthropods and the pathogens they transmit.Ticks Tick Borne Dis. 2018 Jul;9(5):1364-1371. doi: 10.1016/j.ttbdis.2018.05.015. Epub 2018 May 31. Ticks Tick Borne Dis. 2018. PMID: 29886187 Free PMC article. Review.
-
Cultivation of Rickettsia amblyommii in tick cells, prevalence in Florida lone star ticks (Amblyomma americanum).Parasit Vectors. 2014 Jun 13;7:270. doi: 10.1186/1756-3305-7-270. Parasit Vectors. 2014. PMID: 24927809 Free PMC article.
-
The role of autophagy in tick-endosymbiont interactions: insights from Ixodes scapularis and Rickettsia buchneri.Microbiol Spectr. 2024 Jan 11;12(1):e0108623. doi: 10.1128/spectrum.01086-23. Epub 2023 Dec 1. Microbiol Spectr. 2024. PMID: 38038450 Free PMC article.
References
-
- Baldridge GD, Kurtti TJ, Munderloh UG. Susceptibility of Rickettsia monacensis and Rickettsia peacockii to cecropin A, ceratotoxin A and lysozyme. Curr. Microbiol. 2005;51:233–238. - PubMed
-
- Brinton LP, Burgdorfer W. Fine structure of normal hemocytes in Dermacentor andersoni Stiles (Acari: Ixodidae) J. Parasitol. 1971;57:1110–1127. - PubMed
-
- Burgdorfer W. Hemolymph test: a technique for detection of rickettsiae in ticks. Am. J. Trop. Med. Hyg. 1970;19:1010–1014. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

