Leukemia virus long terminal repeat activates NFkappaB pathway by a TLR3-dependent mechanism
- PMID: 16289658
- PMCID: PMC3808874
- DOI: 10.1016/j.virol.2005.10.003
Leukemia virus long terminal repeat activates NFkappaB pathway by a TLR3-dependent mechanism
Abstract
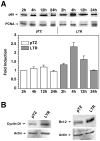
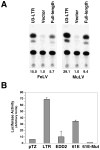
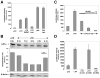
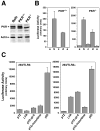
The long terminal repeat (LTR) region of leukemia viruses plays a critical role in tissue tropism and pathogenic potential of the viruses. We have previously reported that U3-LTR from Moloney murine and feline leukemia viruses (Mo-MuLV and FeLV) upregulates specific cellular genes in trans in an integration-independent way. The U3-LTR region necessary for this action does not encode a protein but instead makes a specific RNA transcript. Because several cellular genes transactivated by the U3-LTR can also be activated by NFkappaB, and because the antiapoptotic and growth promoting activities of NFkappaB have been implicated in leukemogenesis, we investigated whether FeLV U3-LTR can activate NFkappaB signaling. Here, we demonstrate that FeLV U3-LTR indeed upregulates the NFkappaB signaling pathway via activation of Ras-Raf-IkappaB kinase (IKK) and degradation of IkappaB. LTR-mediated transcriptional activation of genes did not require new protein synthesis suggesting an active role of the LTR transcript in the process. Using Toll-like receptor (TLR) deficient HEK293 cells and PKR(-/-) mouse embryo fibroblasts, we further demonstrate that although dsRNA-activated protein kinase R (PKR) is not necessary, TLR3 is required for the activation of NFkappaB by the LTR. Our study thus demonstrates involvement of a TLR3-dependent but PKR-independent dsRNA-mediated signaling pathway for NFkappaB activation and thus provides a new mechanistic explanation of LTR-mediated cellular gene transactivation.
Figures


References
-
- Abujamra AL, Faller DV, Ghosh SK. Mutations that abrogate transactivational activity of the feline leukemia virus long terminal repeat do not affect virus replication. Virology. 2003;309(2):294–305. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

