The cell cycle-apoptosis connection revisited in the adult brain
- PMID: 16291699
- PMCID: PMC2171559
- DOI: 10.1083/jcb.200505072
The cell cycle-apoptosis connection revisited in the adult brain
Abstract
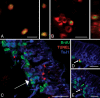
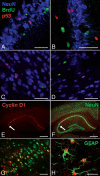
Adult neurogenesis is studied in vivo using thymidine analogues such as bromodeoxyuridine (BrdU) to label DNA synthesis during the S phase of the cell cycle. However, BrdU may also label DNA synthesis events not directly related to cell proliferation, such as DNA repair and/or abortive reentry into the cell cycle, which can occur as part of an apoptotic process in postmitotic neurons. In this study, we used three well-characterized models of injury-induced neuronal apoptosis and the combined visualization of cell birth (BrdU labeling) and death (Tdt-mediated dUTP-biotin nick end labeling) to investigate the specificity of BrdU incorporation in the adult mouse brain in vivo. We present evidence that BrdU is not significantly incorporated during DNA repair and that labeling is not detected in vulnerable or dying postmitotic neurons, even when a high dose of BrdU is directly infused into the brain. These findings have important implications for a controversy surrounding adult neurogenesis: the connection between cell cycle reactivation and apoptosis of terminally differentiated neurons.
Figures





References
-
- Alvarez-Dolado, M., R. Pardal, J.M. Garcia-Verdugo, J.R. Fike, H.O. Lee, K. Pfeffer, C. Lois, S.J. Morrison, and A. Alvarez-Buylla. 2003. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 425:968–973. - PubMed
-
- Bauer, S., M. Hay, B. Amilhon, A. Jean, and E. Moyse. 2005. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience. 130:75–90. - PubMed
-
- Becker, E.B., and A. Bonni. 2004. Cell cycle regulation of neuronal apoptosis in development and disease. Prog. Neurobiol. 72:1–25. - PubMed

