Wnt3a links left-right determination with segmentation and anteroposterior axis elongation
- PMID: 16291790
- PMCID: PMC1389788
- DOI: 10.1242/dev.02149
Wnt3a links left-right determination with segmentation and anteroposterior axis elongation
Abstract
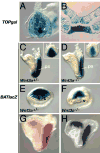
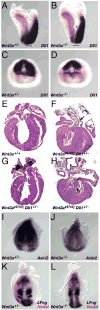
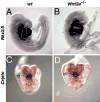
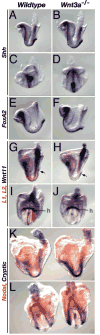
The alignment of the left-right (LR) body axis relative to the anteroposterior (AP) and dorsoventral (DV) axes is central to the organization of the vertebrate body plan and is controlled by the node/organizer. Somitogenesis plays a key role in embryo morphogenesis as a principal component of AP elongation. How morphogenesis is coupled to axis specification is not well understood. We demonstrate that Wnt3a is required for LR asymmetry. Wnt3a activates the Delta/Notch pathway to regulate perinodal expression of the left determinant Nodal, while simultaneously controlling the segmentation clock and the molecular oscillations of the Wnt/beta-catenin and Notch pathways. We provide evidence that Wnt3a, expressed in the primitive streak and dorsal posterior node, acts as a long-range signaling molecule, directly regulating target gene expression throughout the node and presomitic mesoderm. Wnt3a may also modulate the symmetry-breaking activity of mechanosensory cilia in the node. Thus, Wnt3a links the segmentation clock and AP axis elongation with key left-determining events, suggesting that Wnt3a is an integral component of the trunk organizer.
Figures







References
-
- Aulehla A, Herrmann BG. Segmentation in vertebrates: clock and gradient finally joined. Genes Dev. 2004;18:2060–2067. - PubMed
-
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. - PubMed
-
- Barrantes IB, Elia AJ, Wunsch K, Hrabe de Angelis MH, Mak TW, Rossant J, Conlon RA, Gossler A, de la Pompa JL. Interaction between Notch signalling and Lunatic fringe during somite boundary formation in the mouse. Curr Biol. 1999;9:470–480. - PubMed
-
- Beddington RS. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. - PubMed
-
- Bouillet P, Oulad-Abdelghani M, Ward SJ, Bronner S, Chambon P, Dolle P. A new mouse member of the Wnt gene family, mWnt-8, is expressed during early embryogenesis and is ectopically induced by retinoic acid. Mech Dev. 1996;58:141–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

