Do we know what the early visual system does?
- PMID: 16291931
- PMCID: PMC6725861
- DOI: 10.1523/JNEUROSCI.3726-05.2005
Do we know what the early visual system does?
Abstract
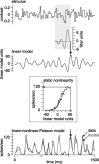
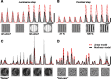
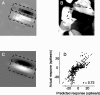
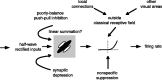
We can claim that we know what the visual system does once we can predict neural responses to arbitrary stimuli, including those seen in nature. In the early visual system, models based on one or more linear receptive fields hold promise to achieve this goal as long as the models include nonlinear mechanisms that control responsiveness, based on stimulus context and history, and take into account the nonlinearity of spike generation. These linear and nonlinear mechanisms might be the only essential determinants of the response, or alternatively, there may be additional fundamental determinants yet to be identified. Research is progressing with the goals of defining a single "standard model" for each stage of the visual pathway and testing the predictive power of these models on the responses to movies of natural scenes. These predictive models represent, at a given stage of the visual pathway, a compact description of visual computation. They would be an invaluable guide for understanding the underlying biophysical and anatomical mechanisms and relating neural responses to visual perception.
Figures



References
-
- Adelson EH, Bergen JR (1985) Spatiotemporal energy models for the perception of motion. J Opt Soc Am A 2: 284-299. - PubMed
-
- Albrecht DG, Geisler WS (1991) Motion selectivity and the contrast-response function of simple cells in the visual cortex. Vis Neurosci 7: 531-546. - PubMed
-
- Albrecht DG, Hamilton DB (1982) Striate cortex of monkey and cat: contrast response function. J Neurophysiol 48: 217-237. - PubMed
-
- Albright TD, Stoner GR (2002) Contextual influences on visual processing. Annu Rev Neurosci 25: 339-379. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
