Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer's disease brain but not in transgenic mouse brain
- PMID: 16291932
- PMCID: PMC6725842
- DOI: 10.1523/JNEUROSCI.2990-05.2005
Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer's disease brain but not in transgenic mouse brain
Abstract
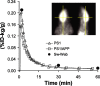
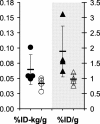
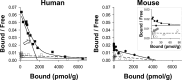
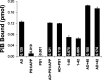
During the development of in vivo amyloid imaging agents, an effort was made to use micro-positron emission tomography (PET) imaging in the presenilin-1 (PS1)/amyloid precursor protein (APP) transgenic mouse model of CNS amyloid deposition to screen new compounds and further study Pittsburgh Compound-B (PIB), a PET tracer that has been shown to be retained well in amyloid-containing areas of Alzheimer's disease (AD) brain. Unexpectedly, we saw no significant retention of PIB in this model even at 12 months of age when amyloid deposition in the PS1/APP mouse typically exceeds that seen in AD. This study describes a series of ex vivo and postmortem in vitro studies designed to explain this low retention. Ex vivo brain pharmacokinetic studies confirmed the low in vivo PIB retention observed in micro-PET experiments. In vitro binding studies showed that PS1/APP brain tissue contained less than one high-affinity (K(d) = 1-2 nm) PIB binding site per 1000 molecules of amyloid-beta (Abeta), whereas AD brain contained >500 PIB binding sites per 1000 molecules of Abeta. Synthetic Abeta closely resembled PS1/APP brain in having less than one high-affinity PIB binding site per 1000 molecules of Abeta, although the characteristics of the few high-affinity PIB binding sites found on synthetic Abeta were very similar to those found in AD brain. We hypothesize that differences in the time course of deposition or tissue factors present during deposition lead to differences in secondary structure between Abeta deposited in AD brain and either synthetic Abeta or Abeta deposited in PS1/APP brain.
Figures



References
-
- Cherry SR (2001) Fundamentals of positron emission tomography and applications in preclinical drug development. J Clin Pharmacol 41: 482-491. - PubMed
-
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S (1996) Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 96: 710-713. - PubMed
-
- Guidotti A, Baraldi M, Schwartz JP, Costa E (1979) Molecular mechanisms regulating the interactions between the benzodazepines and GABA receptors in the central nervous system. Pharmacol Biochem Behav 10: 803-807. - PubMed
-
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4: 97-100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
