Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo
- PMID: 16291946
- PMCID: PMC6725839
- DOI: 10.1523/JNEUROSCI.2250-05.2005
Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo
Abstract
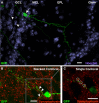
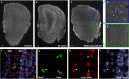
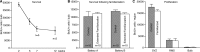
Neurogenesis continues throughout adulthood in the mammalian olfactory bulb and hippocampal dentate gyrus, suggesting the hypothesis that recently generated, adult-born neurons contribute to neural plasticity and learning. To explore this hypothesis, we examined whether olfactory experience modifies the responses of adult-born neurons to odorants, using immediate early genes (IEGs) to assay the response of olfactory granule neurons. We find that, shortly after they differentiate and synaptically integrate, the population of adult-born olfactory granule neurons has a greater population IEG response to novel odors than mature, preexisting neurons. Familiarizing mice with test odors increases the response of the recently incorporated adult-born neuron population to the test odors, and this increased responsiveness is long lasting, demonstrating that the response of the adult-born neuron population is altered by experience. In contrast, familiarizing mice with test odors decreases the IEG response of developmentally generated neurons, suggesting that recently generated adult-born neurons play a distinct role in olfactory processing. The increased IEG response is stimulus specific; familiarizing mice with a set of different, "distractor" odors does not increase the adult-born neuron population response to the test odors. Odor familiarization does not influence the survival of adult-born neurons, indicating that the changes in the population response of adult-born neurons are not attributable to increased survival of odor-stimulated neurons. These results demonstrate that recently generated adult-born olfactory granule neurons and older, preexisting granule neurons undergo contrasting experience-dependent modifications in vivo and support the hypothesis that adult-born neurons are involved in olfactory learning.
Figures


Similar articles
-
Adult-born neurons boost odor-reward association.Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):2514-2519. doi: 10.1073/pnas.1716400115. Epub 2018 Feb 21. Proc Natl Acad Sci U S A. 2018. PMID: 29467284 Free PMC article.
-
Microglial depletion disrupts normal functional development of adult-born neurons in the olfactory bulb.Elife. 2020 Mar 9;9:e50531. doi: 10.7554/eLife.50531. Elife. 2020. PMID: 32150529 Free PMC article.
-
Role of Adult-Born Versus Preexisting Neurons Born at P0 in Olfactory Perception in a Complex Olfactory Environment in Mice.Cereb Cortex. 2020 Mar 21;30(2):534-549. doi: 10.1093/cercor/bhz105. Cereb Cortex. 2020. PMID: 31216001
-
Newborn neurons in the adult olfactory bulb: unique properties for specific odor behavior.Behav Brain Res. 2012 Feb 14;227(2):480-9. doi: 10.1016/j.bbr.2011.08.001. Epub 2011 Aug 6. Behav Brain Res. 2012. PMID: 21843557 Review.
-
Olfactory maps and odor images.Curr Opin Neurobiol. 2002 Aug;12(4):387-92. doi: 10.1016/s0959-4388(02)00348-3. Curr Opin Neurobiol. 2002. PMID: 12139985 Review.
Cited by
-
Linking adult olfactory neurogenesis to social behavior.Front Neurosci. 2012 Nov 30;6:173. doi: 10.3389/fnins.2012.00173. eCollection 2012. Front Neurosci. 2012. PMID: 23226115 Free PMC article.
-
Pair-bonding and social experience modulate new neurons survival in adult male and female prairie voles (Microtus ochrogaster).Front Neuroanat. 2022 Sep 15;16:987229. doi: 10.3389/fnana.2022.987229. eCollection 2022. Front Neuroanat. 2022. PMID: 36189119 Free PMC article.
-
Olfactory discrimination ability of CD-1 mice for a large array of enantiomers.Neuroscience. 2007 Jan 5;144(1):295-301. doi: 10.1016/j.neuroscience.2006.08.063. Epub 2006 Oct 11. Neuroscience. 2007. PMID: 17045753 Free PMC article.
-
The role of calretinin-expressing granule cells in olfactory bulb functions and odor behavior.Sci Rep. 2018 Jun 20;8(1):9385. doi: 10.1038/s41598-018-27692-8. Sci Rep. 2018. PMID: 29925844 Free PMC article.
-
Chronic unpredictable mild stress alters odor hedonics and adult olfactory neurogenesis in mice.Front Neurosci. 2023 Aug 3;17:1224941. doi: 10.3389/fnins.2023.1224941. eCollection 2023. Front Neurosci. 2023. PMID: 37600017 Free PMC article.
References
-
- Allingham K, Brennan PA, Distel H, Hudson R (1999) Expression of c-fos in the main olfactory bulb of neonatal rabbits in response to garlic as a novel and conditioned odour. Behav Brain Res 104: 157-167. - PubMed
-
- Altman J (1969) Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433-457. - PubMed
-
- Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319-335. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8: 963-970. - PubMed
-
- Baba K, Ikeda M, Houtani T, Nakagawa H, Ueyama T, Sato K, Sakuma S, Yamashita T, Tsukahara Y, Sugimoto T (1997) Odor exposure reveals non-uniform expression profiles of c-Jun protein in rat olfactory bulb neurons. Brain Res 774: 142-148. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
