Dynamics of receptor/G protein coupling in living cells
- PMID: 16292347
- PMCID: PMC1356310
- DOI: 10.1038/sj.emboj.7600870
Dynamics of receptor/G protein coupling in living cells
Abstract
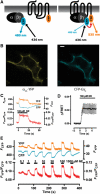
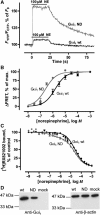
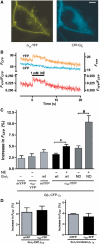
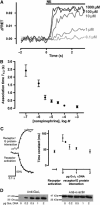
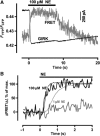
The interaction of activated G protein-coupled receptors with G proteins is a key event in signal transduction. Here, using a fluorescence resonance energy transfer (FRET)-based assay, we measure directly and in living cells the interaction of YFP-labeled alpha(2A)-adrenergic receptors with CFP-labeled G proteins. Upon agonist stimulation, a small, concentration-dependent increase in FRET was observed. No specific basal FRET was detected in the absence of agonist. Kinetics of the onset of receptor/G protein interaction were <100 ms and depended on expression levels of Galpha. Simultaneously recorded G protein-regulated inwardly rectifying K(+) channel currents revealed a maximal current response already at agonist concentrations producing submaximal FRET amplitudes. By analyzing FRET signals in the presence of a Galpha mutant, which dissociates more slowly from activated receptors, it was demonstrated that only a fraction of wild-type G proteins interacts with the activated receptor at any time. Our data suggest that alpha(2A)-adrenergic receptors and G proteins interact by rapid collision coupling and indicate that there is no significant precoupling between these receptors and G proteins.
Figures
References
-
- Azpiazu I, Gautam N (2004) A FRET based sensor indicates that receptor access to a G protein is unrestricted in a living mammalian cell. J Biol Chem 279: 27709–27718 - PubMed
-
- Bourne HR (1997) How receptors talk to trimeric G proteins. Curr Opin Cell Biol 9: 134–142 - PubMed
-
- Bünemann M, Bücheler MM, Philipp M, Lohse MJ, Hein L (2001) Activation and deactivation kinetics of α2A- and α2C-adrenergic receptor-activated G protein-activated inwardly rectifying K+ channel currents. J Biol Chem 276: 47512–47517 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

