A MAPK/HNRPK pathway controls BCR/ABL oncogenic potential by regulating MYC mRNA translation
- PMID: 16293596
- PMCID: PMC1895740
- DOI: 10.1182/blood-2005-09-3732
A MAPK/HNRPK pathway controls BCR/ABL oncogenic potential by regulating MYC mRNA translation
Abstract
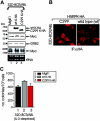
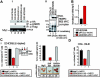
Altered mRNA translation is one of the effects exerted by the BCR/ABL oncoprotein in the blast crisis phase of chronic myelogenous leukemia (CML). Here, we report that in BCR/ABL+ cell lines and in patient-derived CML blast crisis mononuclear and CD34+ cells, p210(BCR/ABL) increases expression and activity of the transcriptional-inducer and translational-regulator heterogeneous nuclear ribonucleoprotein K (hnRNP K or HNRPK) in a dose- and kinase-dependent manner through the activation of the MAPK(ERK1/2) pathway. Furthermore, HNRPK down-regulation and interference with HNRPK translation-but not transcription-regulatory activity impairs cytokine-independent proliferation, clonogenic potential, and in vivo leukemogenic activity of BCR/ABL-expressing myeloid 32Dcl3 and/or primary CD34+ CML-BC patient cells. Mechanistically, we demonstrate that decreased internal ribosome entry site (IRES)-dependent Myc mRNA translation accounts for the phenotypic changes induced by inhibition of the BCR/ABL-ERK-dependent HNRPK translation-regulatory function. Accordingly, MYC protein but not mRNA levels are increased in the CD34+ fraction of patients with CML in accelerated and blastic phase but not in chronic phase CML patients and in the CD34+ fraction of marrow cells from healthy donors. Thus, BCR/ABL-dependent enhancement of HNRPK translation-regulation is important for BCR/ABL leukemogenesis and, perhaps, it might contribute to blast crisis transformation.
Figures





References
-
- Wong S, Witte ON. The BCR-ABL story: bench to bedside and back. Annu Rev Immunol. 2004;22: 247-306. - PubMed
-
- Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103: 4010-4022. - PubMed
-
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340: 1330-1340. - PubMed
-
- Arlinghaus R, Sun T. Signal transduction pathways in Bcr-Abl transformed cells. Cancer Treat Res. 2004;119: 239-270. - PubMed
-
- Gingras AC, Raught B, Sonenberg N. mTOR signaling to translation. Curr Top Microbiol Immunol. 2004;279: 169-197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

