Distinct rhythm generators for inspiration and expiration in the juvenile rat
- PMID: 16293645
- PMCID: PMC1464316
- DOI: 10.1113/jphysiol.2005.098848
Distinct rhythm generators for inspiration and expiration in the juvenile rat
Abstract
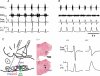
Inspiration and active expiration are commonly viewed as antagonistic phases of a unitary oscillator that generates respiratory rhythm. This view conflicts with observations we report here in juvenile rats, where by administration of fentanyl, a selective mu-opiate agonist, and induction of lung reflexes, we separately manipulated the frequency of inspirations and expirations. Moreover, completely transecting the brainstem at the caudal end of the facial nucleus abolished active expirations, while rhythmic inspirations continued. We hypothesize that inspiration and expiration are generated by coupled, anatomically separate rhythm generators, one generating active expiration located close to the facial nucleus in the region of the retrotrapezoid nucleus/parafacial respiratory group, the other generating inspiration located more caudally in the preBötzinger Complex.
Figures
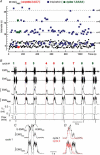
 ) and 100 E-only cycles (mean duration of E-only cycles
) and 100 E-only cycles (mean duration of E-only cycles  ). B, recording of epoch shown in A (∼(150 s, 190 s); green squares represent normal, i.e. I-E cycles (1, 3, 6, 8, 9), and red circles represent E-only cycles (2, 4, 5, 7). Traces from top to bottom: cycle number, EMGGG, EMGABD, their integrals (∫EMGGG and ∫EMGABD), VT and respiratory flow. Vertical dotted lines mark the onset of each EMGABD burst. E-only cycles differed from I-E cycles; see C, D and text. C, ∫EMGGG and ∫EMGABD on an expanded time scale to show pattern of bursting in an I-E cycle (cycle 1); note that ∫EMGGG has two distinct components (I-EMGGG and E-EMGGG) and can have no delay between them or be separate, e.g. Fig. 3, paired vertical lines. EMGABD activity has a two-burst structure. D, comparison of an I-E cycle (cycle 1) and an E-only cycle (cycle 2, red).
). B, recording of epoch shown in A (∼(150 s, 190 s); green squares represent normal, i.e. I-E cycles (1, 3, 6, 8, 9), and red circles represent E-only cycles (2, 4, 5, 7). Traces from top to bottom: cycle number, EMGGG, EMGABD, their integrals (∫EMGGG and ∫EMGABD), VT and respiratory flow. Vertical dotted lines mark the onset of each EMGABD burst. E-only cycles differed from I-E cycles; see C, D and text. C, ∫EMGGG and ∫EMGABD on an expanded time scale to show pattern of bursting in an I-E cycle (cycle 1); note that ∫EMGGG has two distinct components (I-EMGGG and E-EMGGG) and can have no delay between them or be separate, e.g. Fig. 3, paired vertical lines. EMGABD activity has a two-burst structure. D, comparison of an I-E cycle (cycle 1) and an E-only cycle (cycle 2, red).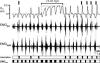



Comment in
-
Novel two-rhythm generator theory of breathing in mammals.J Physiol. 2006 Jan 15;570(Pt 2):207. doi: 10.1113/jphysiol.2005.101709. Epub 2005 Nov 24. J Physiol. 2006. PMID: 16308345 Free PMC article. No abstract available.
References
-
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. - PubMed
-
- Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharmacol. 2004;2:221–243. - PubMed
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem–spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. - PubMed
-
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. - PubMed
-
- Borday C, Wrobel L, Fortin G, Champagnat J, Thaeron-Antono C, Thoby-Brisson M. Developmental gene control of brainstem function: views from the embryo. Prog Biophys Mol Biol. 2004;84:89–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

