Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin
- PMID: 16294219
- PMCID: PMC1283939
- DOI: 10.1172/JCI25040
Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin
Abstract
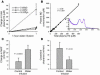
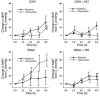
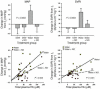
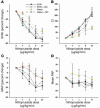
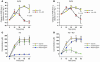
During intravascular hemolysis in human disease, vasomotor tone and organ perfusion may be impaired by the increased reactivity of cell-free plasma hemoglobin (Hb) with NO. We experimentally produced acute intravascular hemolysis in a canine model in order to test the hypothesis that low levels of decompartmentalized or cell-free plasma Hb will severely reduce NO bioavailability and produce vasomotor instability. Importantly, in this model the total intravascular Hb level is unchanged; only the compartmentalization of Hb within the erythrocyte membrane is disrupted. Using a full-factorial design, we demonstrate that free water-induced intravascular hemolysis produces dose-dependent systemic vasoconstriction and impairs renal function. We find that these physiologic changes are secondary to the stoichiometric oxidation of endogenous NO by cell-free plasma oxyhemoglobin. In this model, 80 ppm of inhaled NO gas oxidized 85-90% of plasma oxyhemoglobin to methemoglobin, thereby inhibiting endogenous NO scavenging by cell-free Hb. As a result, the vasoconstriction caused by acute hemolysis was attenuated and the responsiveness to systemically infused NO donors was restored. These observations confirm that the acute toxicity of intravascular hemolysis occurs secondarily to the accelerated dioxygenation reaction of plasma oxyhemoglobin with endothelium-derived NO to form bioinactive nitrate. These biochemical and physiological studies demonstrate a major role for the intact erythrocyte in NO homeostasis and provide mechanistic support for the existence of a human syndrome of hemolysis-associated NO dysregulation, which may contribute to the vasculopathy of hereditary, acquired, and iatrogenic hemolytic states.
Figures

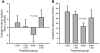

References
-
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. - PubMed
-
- Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ. Res. 1987;61:866–879. - PubMed
-
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. - PubMed
-
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

