Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia
- PMID: 16294224
- PMCID: PMC1283940
- DOI: 10.1172/JCI25461
Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia
Erratum in
- J Clin Invest. 2006 Feb;116(2):549
Abstract
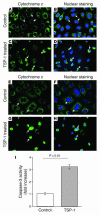
Thrombospondin 1 (TSP-1) is a matricellular protein that inhibits angiogenesis and causes apoptosis in vivo and in vitro in several cancerous cells and tissues. Here we identify TSP-1 as the molecule with the highest induction level at 3 hours of IR injury in rat and mouse kidneys subjected to ischemia/reperfusion (IR) injury using the DNA microarray approach. Northern hybridizations demonstrated that TSP-1 expression was undetectable at baseline, induced at 3 and 12 hours, and returned to baseline levels at 48 hours of reperfusion. Immunocytochemical staining identified the injured proximal tubules as the predominant sites of expression of TSP-1 in IR injury and showed colocalization of TSP-1 with activated caspase-3. Addition of purified TSP-1 to normal kidney proximal tubule cells or cells subjected to ATP depletion in vitro induced injury as demonstrated by cytochrome c immunocytochemical staining and caspase-3 activity. The deleterious role of TSP-1 in ischemic kidney injury was demonstrated directly in TSP-1 null mice, which showed significant protection against IR injury-induced renal failure and tubular damage. We propose that TSP-1 is a novel regulator of ischemic damage in the kidney and may play an important role in the pathophysiology of ischemic kidney failure.
Figures




Similar articles
-
Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2003 May;284(5):F1046-55. doi: 10.1152/ajprenal.00318.2002. Epub 2003 Jan 28. Am J Physiol Renal Physiol. 2003. PMID: 12554636
-
Deletion of the kinase domain in death-associated protein kinase attenuates tubular cell apoptosis in renal ischemia-reperfusion injury.J Am Soc Nephrol. 2004 Jul;15(7):1826-34. doi: 10.1097/01.asn.0000131527.59781.f2. J Am Soc Nephrol. 2004. PMID: 15213270
-
Cloning and expression of rat caspase-6 and its localization in renal ischemia/reperfusion injury.Kidney Int. 2002 Jul;62(1):106-15. doi: 10.1046/j.1523-1755.2002.00427.x. Kidney Int. 2002. PMID: 12081569
-
Thrombospondin in renal disease.Nephron Exp Nephrol. 2009;111(3):e61-6. doi: 10.1159/000198235. Epub 2009 Jan 31. Nephron Exp Nephrol. 2009. PMID: 19182492 Review.
-
The cell biology of thrombospondin-1.Matrix Biol. 2000 Dec;19(7):597-614. doi: 10.1016/s0945-053x(00)00107-4. Matrix Biol. 2000. PMID: 11102749 Review.
Cited by
-
Podocyte-Specific Induction of Krüppel-Like Factor 15 Restores Differentiation Markers and Attenuates Kidney Injury in Proteinuric Kidney Disease.J Am Soc Nephrol. 2018 Oct;29(10):2529-2545. doi: 10.1681/ASN.2018030324. Epub 2018 Aug 24. J Am Soc Nephrol. 2018. PMID: 30143559 Free PMC article.
-
Regulated cell death in AKI.J Am Soc Nephrol. 2014 Dec;25(12):2689-701. doi: 10.1681/ASN.2014030262. Epub 2014 Jun 12. J Am Soc Nephrol. 2014. PMID: 24925726 Free PMC article. Review.
-
The counteradhesive proteins, thrombospondin 1 and SPARC/osteonectin, open the tyrosine phosphorylation-responsive paracellular pathway in pulmonary vascular endothelia.Microvasc Res. 2009 Jan;77(1):13-20. doi: 10.1016/j.mvr.2008.08.008. Epub 2008 Oct 1. Microvasc Res. 2009. PMID: 18952113 Free PMC article. Review.
-
Transcriptional analysis of infiltrating T cells in kidney ischemia-reperfusion injury reveals a pathophysiological role for CCR5.Am J Physiol Renal Physiol. 2012 Mar 15;302(6):F762-73. doi: 10.1152/ajprenal.00335.2011. Epub 2011 Dec 7. Am J Physiol Renal Physiol. 2012. PMID: 22160774 Free PMC article.
-
Capillary rarefaction and altered renal development: the imbalance between pro- and anti-angiogenic factors in response to angiotensin II inhibition in the developing rat kidney.J Mol Histol. 2018 Apr;49(2):219-228. doi: 10.1007/s10735-018-9762-7. Epub 2018 Feb 13. J Mol Histol. 2018. PMID: 29442209
References
-
- Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–1178. - PubMed
-
- Tritto I, et al. A short burst of oxygen radicals at reflow induces sustained release of oxidized glutathione from postischemic hearts. Free Radic. Biol. Med. 1998;24:290–297. - PubMed
-
- Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin. Nephrol. 1998;18:490–497. - PubMed
-
- Rabb H, Wang Z, Postler G, Soleimani M. Possible molecular basis for changes in potassium handling in acute renal failure. Am. J. Kidney Dis. 2000;35:871–877. - PubMed
-
- Sheridan AM, Bonventre JV. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr. Opin. Nephrol. Hypertens. 2000;9:427–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

