Periocular routes for retinal drug delivery
- PMID: 16296723
- PMCID: PMC11864216
- DOI: 10.1517/17425247.1.1.99
Periocular routes for retinal drug delivery
Abstract
Despite numerous scientific efforts, delivery of therapeutic amounts of a drug to the retina remains a challenge. This challenge is compounded if chronic therapy is desired. The inability or inefficiency of topical and systemic routes for retinal delivery of existing drugs is now widely accepted. Although the intravitreal route offers high local concentrations in the vitreous and, hence, retina, these advantages are offset by side effects, such as cataracts, endophthalmitis and retinal detachment, following repeated intravitreal injections, or intravitreal placement of sustained-release implants. As discussed in this review, periocular routes, including subconjunctival, sub-tenon, retrobulbar, peribulbar and posterior juxtascleral routes, potentially offer a more promising alternative for enhanced drug delivery to the retina compared with topical and systemic routes. Periocular routes exploit the permeability of sclera for retinal drug delivery, and they are particularly useful for administering sustained-release systems of potent drugs. This review discusses the various periocular routes with respect to their anatomical location, pharmacokinetics, safety and mechanisms of drug delivery. In the coming years, several innovations in absorption enhancement, drug delivery systems and drug administration devices are anticipated for improving retinal drug delivery via periocular routes.
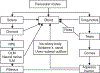
Figures





References
-
- KOMPELLA UB, LEE VHL: Barriers to drug transport in ocular epithelia: In: Transport processes in pharmaceutical systems. Amidon GL, Lee PI, Topp EM (Eds), Marcel Dekker, Inc., New York, USA: (1999):317–376.
-
- SUNKARA G, KOMPELLA UB: Membrane transport processes in the eye. (Second) Ocular Drug Delivery Systems. Mitra AK (Ed.), Marcel Dekker, Inc., New York, USA: (2003):13–58.
-
- DENNINGHOFF KR, SMITH MH, LOMPADO A, HILLMAN LW: Retinal venous oxygen saturation and cardiac output during controlled hemorrhage and resuscitation. J. Appl. Physiol. (2003) 94(3):891–896. - PubMed
-
- RENNIE IG: Clincally important reactions to systemic drug therapy. Drug Saf. (1993) 9(3):196–211. - PubMed
-
- DAVIDSON SI, RENNIE IG: Ocular toxicity from systemic drug therapy. An overview of clinically important adverse reactions. Med. Toxicol. (1986) 1(3):217–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
