The immune responses to human and microbial heat shock proteins in periodontal disease with and without coronary heart disease
- PMID: 16297172
- PMCID: PMC1809534
- DOI: 10.1111/j.1365-2249.2005.02953.x
The immune responses to human and microbial heat shock proteins in periodontal disease with and without coronary heart disease
Abstract
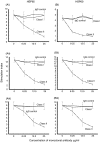
The human 60 kDa and microbial 65 kDa heat shock proteins (HSP) have been implicated in the pathogenesis of chronic periodontitis (P) and coronary heart disease (CHD). We have studied four male non-smoking cohorts of 81 subjects, matched for age. Group (a) consisted of a healthy group with minimal gingivitis (n = 18), group (b) were patients with P (n = 23), group (c) patients with CHD and minimal gingivitis (n = 20) and group (d) patients with CHD and P (n = 20). T cells separated from peripheral blood were found to be primed to both microbial HSP65 and human HSP60 but significant CD4, human leucocyte antigen (HLA) class II-restricted proliferative responses were found only with the human HSP60 in patients with P (P < 0.001) and CHD without (P < 0.001) or with (P < 0.00001) periodontitis. Dose-dependent inhibition of T cell proliferative responses was carried out to determine the receptors involved in recognition of HSP60 and HSP65. Monoclonal antibodies to CD14 showed inhibition of T cell proliferation stimulated by both HSP60 and HSP65, consistent with the role of CD14 as a receptor for these HSPs in P and CHD. The toll-like receptor 2 (TLR-) and TLR-4 were then studied and these showed that TLR-4 was recognized by microbial HSP65, whereas TLR-2 was recognised by human HSP60 in both P and CHD. However, a dissociation was found in the HSP60 and TLR4 interaction, as TLR4 appeared to have been recognized by HSP60 in P but not in CHD. The results suggest an autoimmune or cross-reactive CD4(+) class II-restricted T cell response to the human HSP60 in P and CHD. Further studies are required to determine if there is a common epitope within HSP60 that stimulates T cell proliferation in P and CHD.
Figures




Similar articles
-
Responses to gram negative enteric bacterial antigens by synovial T cells from patients with juvenile chronic arthritis: recognition of heat shock protein HSP60.J Rheumatol. 1993 Aug;20(8):1388-96. J Rheumatol. 1993. PMID: 7693941
-
Pathogenic role of tonsillar lymphocytes in associated with HSP60/65 in Pustulosis palmaris et plantaris.Auris Nasus Larynx. 2009 Oct;36(5):578-85. doi: 10.1016/j.anl.2008.11.009. Epub 2009 Mar 5. Auris Nasus Larynx. 2009. PMID: 19268507
-
Inhibition of adjuvant-induced arthritis by DNA vaccination with the 70-kd or the 90-kd human heat-shock protein: immune cross-regulation with the 60-kd heat-shock protein.Arthritis Rheum. 2004 Nov;50(11):3712-20. doi: 10.1002/art.20635. Arthritis Rheum. 2004. PMID: 15529360
-
Heat shock protein 60 and adjuvant arthritis: a model for T cell regulation in human arthritis.Springer Semin Immunopathol. 2003 Aug;25(1):47-63. doi: 10.1007/s00281-003-0128-7. Springer Semin Immunopathol. 2003. PMID: 12904891 Review.
-
Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis.Immunol Rev. 1991 Jun;121:5-28. doi: 10.1111/j.1600-065x.1991.tb00821.x. Immunol Rev. 1991. PMID: 1937534 Review.
Cited by
-
The Protective Action of Piperlongumine Against Mycobacterial Pulmonary Tuberculosis in Its Mitigation of Inflammation and Macrophage Infiltration in Male BALB/c Mice.J Vet Res. 2021 Nov 20;65(4):431-440. doi: 10.2478/jvetres-2021-0061. eCollection 2021 Dec. J Vet Res. 2021. PMID: 35111996 Free PMC article.
-
Periodontal disease as a risk factor for adverse pregnancy outcomes: a systematic review and meta-analysis of case-control studies.Odontology. 2012 Jul;100(2):232-40. doi: 10.1007/s10266-011-0036-z. Epub 2011 Jul 8. Odontology. 2012. PMID: 21739194
-
Relationship of periodontal infection to serum antibody levels to periodontopathic bacteria and inflammatory markers in periodontitis patients with coronary heart disease.Clin Exp Immunol. 2007 Sep;149(3):445-52. doi: 10.1111/j.1365-2249.2007.03450.x. Epub 2007 Jul 23. Clin Exp Immunol. 2007. PMID: 17645769 Free PMC article.
-
Sophora flavescens protects against mycobacterial Trehalose Dimycolate-induced lung granuloma by inhibiting inflammation and infiltration of macrophages.Sci Rep. 2018 Mar 2;8(1):3903. doi: 10.1038/s41598-018-22286-w. Sci Rep. 2018. PMID: 29500453 Free PMC article.
References
-
- Listgarten MA, Loomer PM. Microbial identification in the management of periodontal diseases. A systematic review. Ann Periodontol. 2003;8:182–92. - PubMed
-
- Kumar P, Griffin A, Barton J, Paster B, Moeschberger M, Leys E. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82(5):338–44. - PubMed
-
- Thole JE, Hindersson P, de Bruyn J, et al. Antigenic relatedness of a strongly immunogenic 65 kDA mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb Pathogen. 1988;4:71–83. - PubMed
-
- Feige U, van Eden W. Infection, autoimmunity and autoimmune disease. EXS. 1996;77:359–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

