Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy
- PMID: 16297189
- PMCID: PMC1360172
- DOI: 10.1111/j.0022-202X.2005.23846.x
Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy
Abstract
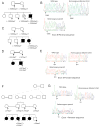
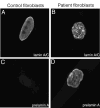
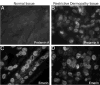
Restrictive dermopathy (RD) is a lethal human genetic disorder characterized by very tight, thin, easily eroded skin, rocker bottom feet, and joint contractures. This disease was recently reported to be associated with a single heterozygous mutation in ZMPSTE24 and hypothesized to be a digenic disorder (Navarro et al, Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet 13:2493-2503, 2004). ZMPSTE24 encodes an enzyme necessary for the correct processing and maturation of lamin A, an intermediate filament component of the nuclear envelope. Here we present four unrelated patients with homozygous mutations in ZMPSTE24 and a fifth patient with compound heterozygous mutations in ZMPSTE24. Two of the three different mutations we found are novel, and all are single base insertions that result in messenger RNA frameshifts. As a consequence of the presumed lack of ZMPSTE24 activity, prelamin A, the unprocessed toxic form of lamin A, was detected in the nuclei of both cultured cells and tissue from RD patients, but not in control nuclei. Abnormally aggregated lamin A/C was also observed. These results indicate that RD is an autosomal recessive laminopathy caused by inactivating ZMPSTE24 mutations that result in defective processing and nuclear accumulation of prelamin A.
Figures
Comment in
-
Lamin processing comes of age.J Invest Dermatol. 2005 Nov;125(5):xii-xiii. doi: 10.1111/j.0022-202X.2005.23871.x. J Invest Dermatol. 2005. PMID: 16297177 No abstract available.
References
-
- Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. - PubMed
-
- Capanni C, Cenni V, Mattioli E, et al. Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy fibroblasts: altered intermolecular interaction with emerin and implications for gene transcription. Exp Cell Res. 2003;291:122–134. - PubMed
-
- Caux F, Dubosclard E, Lascols O, et al. A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab. 2003;88:1006–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

