1H and 13C-NMR and molecular dynamics studies of cyclosporin a interacting with magnesium(II) or cerium(III) in acetonitrile. Conformational changes and cis-trans conversion of peptide bonds
- PMID: 16299069
- PMCID: PMC1367286
- DOI: 10.1529/biophysj.105.074245
1H and 13C-NMR and molecular dynamics studies of cyclosporin a interacting with magnesium(II) or cerium(III) in acetonitrile. Conformational changes and cis-trans conversion of peptide bonds
Abstract
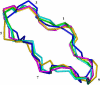
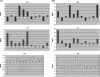
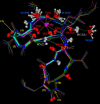
Cyclosporin A (CsA) is an important drug used to prevent graft rejection in organ transplantations. Its immunosuppressive activity is related to the inhibition of T-cell activation through binding with the proteins Cyclophilin (Cyp) and, subsequently, Calcineurin (CN). In the complex with its target (Cyp), CsA adopts a conformation with all trans peptide bonds and this feature is very important for its pharmacological action. Unfortunately, CsA can cause several side effects, and it can favor the excretion of calcium and magnesium. To evaluate the possible role of conformational effects induced by these two metal ions in the action mechanism of CsA, its complexes with Mg(II) and Ce(III) (the latter as a paramagnetic probe for calcium) have been examined by two-dimensional NMR and relaxation rate analysis. The conformations of the two complexes and of the free form have been determined by restrained molecular dynamics calculations based on the experimentally obtained metal-proton and interproton distances. The findings here ratify the formation of 1:1 complexes of CsA with both Mg(II) and Ce(III), with metal coordination taking place on carbonyl oxygens and substantially altering the peptide structure with respect to the free form, although the residues involved and the resulting conformational changes, including cis-trans conversion of peptide bonds, are different for the two metals.
Figures





Similar articles
-
Solution structures of cyclosporin a and its complex with dysprosium(III) in SDS micelles: NMR and molecular dynamics studies.J Phys Chem B. 2008 Jan 24;112(3):828-35. doi: 10.1021/jp076837z. Epub 2007 Dec 21. J Phys Chem B. 2008. PMID: 18095667
-
A proposed molecular model for the interaction of calcineurin with the cyclosporin A-cyclophilin A complex.Bioorg Med Chem. 1999 Jul;7(7):1389-402. doi: 10.1016/s0968-0896(99)00072-3. Bioorg Med Chem. 1999. PMID: 10465413
-
Studies of the interaction of potassium(I), calcium(II), magnesium(II), and copper(II) with cyclosporin A.J Inorg Biochem. 2003 Oct 1;97(2):191-8. doi: 10.1016/s0162-0134(03)00288-5. J Inorg Biochem. 2003. PMID: 14512197
-
Crystal structures of cyclophilin and its partners.Front Biosci. 2004 Sep 1;9:2285-96. doi: 10.2741/1396. Front Biosci. 2004. PMID: 15353287 Review.
-
Some new aspects of molecular mechanisms of cyclosporin A effect on immune response.APMIS. 1995 Jun;103(6):401-15. doi: 10.1111/j.1699-0463.1995.tb01125.x. APMIS. 1995. PMID: 7546642 Review.
Cited by
-
Exploring the Effects of Cyclosporin A to Isocyclosporin A Rearrangement on Ion Mobility Separation.Anal Chem. 2024 Mar 12;96(10):4163-4170. doi: 10.1021/acs.analchem.3c05165. Epub 2024 Mar 2. Anal Chem. 2024. PMID: 38430121 Free PMC article.
-
Novel formulations for antimicrobial peptides.Int J Mol Sci. 2014 Oct 9;15(10):18040-83. doi: 10.3390/ijms151018040. Int J Mol Sci. 2014. PMID: 25302615 Free PMC article. Review.
-
Taming Conformational Heterogeneity on Ion Racetrack to Unveil Principles that Drive Membrane Permeation of Cyclosporines.JACS Au. 2024 Mar 16;4(4):1458-1470. doi: 10.1021/jacsau.4c00011. eCollection 2024 Apr 22. JACS Au. 2024. PMID: 38665661 Free PMC article.
-
Blood-Brain Barrier Permeability Is Exacerbated in Experimental Model of Hepatic Encephalopathy via MMP-9 Activation and Downregulation of Tight Junction Proteins.Mol Neurobiol. 2018 May;55(5):3642-3659. doi: 10.1007/s12035-017-0521-7. Epub 2017 May 18. Mol Neurobiol. 2018. PMID: 28523565
-
DNA aptamers to human immunodeficiency virus reverse transcriptase selected by a primer-free SELEX method: characterization and comparison with other aptamers.Nucleic Acid Ther. 2012 Jun;22(3):162-76. doi: 10.1089/nat.2011.0327. Epub 2012 May 3. Nucleic Acid Ther. 2012. PMID: 22554064 Free PMC article.
References
-
- Ruegger, A., M. Kuhn, H. Lichti, H. R. Loosli, R. Huguenin, C. Quiquerez, and A. von Wartburg. 1976. Cyclosporin A, a peptide metabolite from Trichoderma polysporum Rifai, with a remarkable immunosuppressive activity. Helv. Chim. Acta. 59:1075–1092. - PubMed
-
- Wenger, R. M. 1985. Synthesis of cyclosporine and analogues: structural requirements for immunosuppressive activity. Angew. Chem. 24:77–85. - PubMed
-
- Raghavan, S., and M. A. Rasheed. 2004. Modular and stereoselective formal synthesis of MeBmt, an unusual amino acid constituent of cyclosporin A. Tetrahedron. 60:3059–3065.
-
- Potter, B., R. A. Palmer, R. Withnall, T. C. Jenkins, and B. Z. Chowdhry. 2003. Two new cyclosporin folds observed in the structures of the immunosuppressant cyclosporin G and the formyl peptide receptor antagonist cyclosporin H at ultra-high resolution. Org. Biomol. Chem. 1:1466–1474. - PubMed
-
- Hsu, W., S. L. Heald, M. W. Harding, R. E. Handschumacher, and I. M. Armitage. 1990. Structural elements pertinent to the interaction of cyclosporin A with its specific receptor protein, cyclophilin. Biochem. Pharmacol. 40:131–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

