Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen
- PMID: 16299295
- PMCID: PMC1307056
- DOI: 10.1128/IAI.73.12.8017-8026.2005
Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen
Abstract
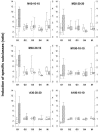
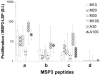
The C-terminal conserved region of Plasmodium falciparum merozoite surface protein 3 (MSP3) is the trigger antigen of a protective immune response mediated by cytophilic antibodies. In an open, randomized, two-adjuvant (Montanide ISA 720, aluminum hydroxide) phase I clinical trial we evaluated the safety and immunogenicity of increasing doses of a long synthetic peptide construct spanning the conserved region of MSP3 targeted by biologically active antibodies (MSP3-LSP). Thirty-five healthy volunteers were randomized to receive three subcutaneous injections on days 0, 30, and 120. Of the 100 injections given, 10 caused severe local reactions, 62 caused transient mild to moderate local reactions, and 28 caused no reaction. On the basis of preestablished exclusion criteria, use of the Montanide formulation led to withdrawal of five volunteers after the second injection. This led to a reduction in the subsequent vaccine doses in four of the groups. No vaccine-related serious adverse events occurred throughout the trial. After the third injection, volunteers displayed a marked specific anti-MSP3-LSP antibody response (23/30 individuals, compared with 29/34 individuals for plasma from an area where malaria is endemic), an anti-native MSP3 antibody response (19/30 individuals), a T-cell-antigen-specific proliferative response (26/30 individuals), and gamma interferon production (25/30 individuals). In conclusion, the MSP3-LSP vaccine was immunogenic with both adjuvants, although it was unacceptably reactogenic when it was combined with Montanide. The potential usefulness of the candidate vaccine is supported by the induction of a strong cytophilic response (i.e., the type of anti-MSP3 antibodies involved in antibody-dependent, monocyte-mediated protective mechanisms in areas where malaria is endemic).
Figures

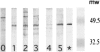


References
-
- Aribot, G., C. Rogier, J. L. Sarthou, J. F. Trape, A. T. Balde, P. Druilhe, and C. Roussilhon. 1996. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa). Am. J. Trop. Med. Hyg. 54:449-457. - PubMed
-
- Astori, M., C. von Garnier, A. Kettner, N. Dufour, G. Corradin, and F. Spertini. 2000. Inducing tolerance by intranasal administration of long peptides in naive and primed CBA/J. mice. J. Immunol. 165:3497-3505. - PubMed
-
- Baylor, N. W., W. Egan, and P. Richman. 2002. Aluminum salts in vaccines—US perspective. Vaccine 20:S18-S23. - PubMed
-
- Blum-Tirouvanziam, U., C. Beghdadi-Rais, M. A. Roggero, D. Valmori, S. Bertholet, C. Bron, N. Fasel, and G. Corradin. 1994. Elicitation of specific cytotoxic T cells by immunization with malaria soluble synthetic polypeptides. J. Immunol. 153:4134-4141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

