Noncapsulated Klebsiella pneumoniae bearing mannose-containing O antigens is rapidly eradicated from mouse lung and triggers cytokine production by macrophages following opsonization with surfactant protein D
- PMID: 16299325
- PMCID: PMC1307026
- DOI: 10.1128/IAI.73.12.8282-8290.2005
Noncapsulated Klebsiella pneumoniae bearing mannose-containing O antigens is rapidly eradicated from mouse lung and triggers cytokine production by macrophages following opsonization with surfactant protein D
Abstract
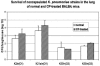
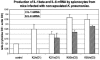
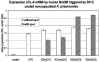
To better understand the relationship between the surface polysaccharides of pulmonary pathogens and components of the lung innate immune system, we employed selected serotypes of Klebsiella pneumoniae expressing distinct capsular polysaccharides and/or O antigen in a murine model of K. pneumoniae infection. In addition, we examined the effect of surfactant protein D (SP-D) on the cytokine response of human monocyte-derived macrophages to these serotypes in vitro. Noncapsulated mannose-containing O3 serotypes (K50/n and K55/n), which react efficiently with SP-D in vitro, triggered high levels of interleukin-1beta (IL-1beta) and IL-6 production. In vivo, they were more efficiently cleared from the lungs of mice but not from macrophage-depleted mice. They also were more efficiently internalized by alveolar macrophages in vivo. In contrast, galactose-containing O1 serotypes (K2/n and K21a/n), which interact poorly with SP-D, exhibited significantly lower cytokine production and less efficient pulmonary clearance and were ineffectively internalized by alveolar macrophages. These findings are consistent with in vitro results showing that production of IL-1beta and IL-6 mRNA and IL-6 protein by human macrophages exposed to mannose-bearing Klebsiella O serotypes is significantly increased by SP-D. Thus, survival of inhaled bacteria in the lung depends partially on the lipopolysaccharide structure of the bacteria and their interactions with innate immunity components. We speculate that an imbalance of host SP-D and therefore cytokine levels may result in high susceptibility of the host to the pathogen.
Figures




References
-
- Aderem, A., and D. M. Underhill. 1999. Mechanism of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-628. - PubMed
-
- Banyer, J. L., N. H. R. Hamilton, I. A. Ramshaw, and A. J. Ramsey. 2000. Cytokines and innate and adaptive immunity. Rev. Immunodiagn. 2:359-373. - PubMed
-
- Bartlett, J. G., O'Keefe, P., F. P. Tally, T. J. Louie, and S. L. Gorbach. 1986. Bacteriology of hospital-acquired pneumonia. Arch. Intern. Med. 146:868-871. - PubMed
-
- Broug-Holub, E., G. B. Toews, J. F. van Iwaarden, R. M. Strieter, S. L. Kunkel, R. Paine III, and T. J. Standiford. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumoniae: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139-1146. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

