Liver cancer: the role of stem cells
- PMID: 16300653
- PMCID: PMC6496116
- DOI: 10.1111/j.1365-2184.2005.00354.x
Liver cancer: the role of stem cells
Abstract
Studies of aggregation chimaeras and X-linked polymorphisms strongly suggest that liver tumours are derived from single cells (monoclonal), but the important question is, which cell? Stem cell biology and cancer are inextricably linked. In continually renewing tissues such as the gut mucosa and epidermis, where a steady flux of cells occurs from the stem cell zone to the terminally differentiated cells that are imminently to be lost, it is widely accepted that cancer is a disease of stem cells, since these are the only cells that persist in the tissue for a sufficient length of time to acquire the requisite number of genetic changes for neoplastic development. In the liver the identity of the founder cells for the two major primary tumours, hepatocellular carcinoma and cholangiocarcinoma, is more problematic. The reason for this is that no such obvious unidirectional flux occurs in the liver, although it is held that the centrilobular hepatocytes may be more differentiated (polyploid) and closer to cell senescence than those cells closest to the portal areas. Moreover, the existence of bipotential hepatic progenitor cells, along with hepatocytes endowed with longevity and long-term repopulating potential suggests there may be more than one type of carcinogen target cell. Cell proliferation at the time of carcinogen exposure is pivotal for 'fixing' any genotoxic injury into a heritable form, thus any proliferative cell in the liver can be susceptible to neoplastic transformation. Hepatocytes are implicated in many instances of hepatocellular carcinoma, direct injury to the biliary epithelium implicates cholangiocytes in some cases of cholangiocarcinoma, while hepatic progenitor cell/oval cell activation accompanies many instances of liver damage irrespective of aetiology, making such cells very likely carcinogen targets. Of course, we must qualify this assertion by stating that many carcinogens are both cytotoxic and cytostatic, and that hepatic progenitor cell proliferation may be merely a bystander effect of this toxicity. An in-depth discussion of causes of cancer in the liver is beyond the scope of this review, but infectious agents (e.g. hepatitis B and C viruses) play a major role, not just in transactivating or otherwise disrupting cellular proto-oncogenes (hepatitis B virus), but also in causing chronic inflammation (hepatitis C and B viruses). Sustained epithelial proliferation in a milieu rich in inflammatory cells, growth factors and DNA-damaging agents (reactive oxygen and nitrogen species--produced to fight infection), will lead to permanent genetic changes in proliferating cells. Up-regulation of the transcription factor NF-kappaB in transformed hepatocytes, through the paracrine action of TNF-alpha from neighbouring endothelia and inflammatory cells, may be critical for tumour progression given the mitogenic and antiapoptotic properties of proteins encoded by many of NF-kappaB's target genes.
Figures
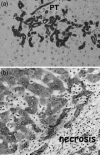




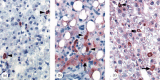
References
-
- Alison MR (2003) Characterization of the differentiation capacity of rat‐derived hepatic stem cells. Semin. Liver Dis. 23, 325. - PubMed
-
- Alison MR, Golding M, Sarraf CE, Edwards R, Lalani EN (1996) Liver damage in the rat induces hepatocyte stem cells from biliary epithelial cells. Gastroenterology 110, 1182. - PubMed
-
- Avril A, Pichard V, Bralet MP, Ferry N (2004) Mature hepatocytes are the source of small hepatocyte‐like progenitor cells in the retrorsine model of liver injury. J. Hepatol. 41, 737. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

