Ephrin-as guide the formation of functional maps in the visual cortex
- PMID: 16301175
- PMCID: PMC2424263
- DOI: 10.1016/j.neuron.2005.10.026
Ephrin-as guide the formation of functional maps in the visual cortex
Abstract
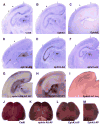
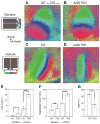
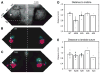
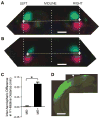
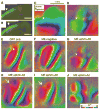
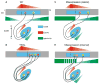
Ephrin-As and their receptors, EphAs, are expressed in the developing cortex where they may act to organize thalamic inputs. Here, we map the visual cortex (V1) in mice deficient for ephrin-A2, -A3, and -A5 functionally, using intrinsic signal optical imaging and microelectrode recording, and structurally, by anatomical tracing of thalamocortical projections. V1 is shifted medially, rotated, and compressed and its internal organization is degraded. Expressing ephrin-A5 ectopically by in utero electroporation in the lateral cortex shifts the map of V1 medially, and expression within V1 disrupts its internal organization. These findings indicate that interactions between gradients of EphA/ephrin-A in the cortex guide map formation, but that factors other than redundant ephrin-As are responsible for the remnant map. Together with earlier work on the retinogeniculate map, the current findings show that the same molecular interactions may operate at successive stages of the visual pathway to organize maps.
Figures


Comment in
-
Local axon guidance in cerebral cortex and thalamus: are we there yet?Neuron. 2005 Nov 23;48(4):522-4. doi: 10.1016/j.neuron.2005.11.011. Neuron. 2005. PMID: 16301165
References
-
- Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. - PubMed
-
- Bolz J, Uziel D, Muhlfriedel S, Gullmar A, Peuckert C, Zarbalis K, Wurst W, Torii M, Levitt P. Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. J Neurobiol. 2004;59:82–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

