A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection
- PMID: 16301662
- PMCID: PMC3514507
- DOI: 10.4049/jimmunol.175.11.7536
A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection
Abstract
Acquired immunity to murine Chlamydia trachomatis genital tract reinfection has long been assumed to be solely dependent on cell-mediated immunity. However, in this study, we identify a previously unrecognized protective role for Ab. Immunity develops in Ab-deficient mice following the resolution of primary chlamydial genital infection. Subsequent depletion of CD4+ T cells, but not CD8+ T cells, in those immune Ab-deficient mice before secondary infectious challenge, resulted in an infection that did not resolve. Passive immunization with immune (convalescent) serum conferred a marked level of protective immunity to reinfection, which was characterized by a striking decrease in bacterial shedding, from >100,000 inclusion forming units to fewer than 10 inclusion forming units, and a shortened duration of infection. Furthermore, mAbs to the chlamydial major outer membrane protein and LPS conferred significant levels of immunity to reinfection and reduced chlamydial shedding by >100-fold. Anti-heat shock protein 60 mAb had no protective effect. In contrast to the marked protective efficacy of immune serum on reinfection, the course of primary infection was essentially unaltered by the passive transfer of immune serum. Our results convincingly demonstrate that Abs contribute importantly to immunity to chlamydial genital tract reinfection, and that Ab-mediated protection is highly dependent on CD4+ T cell-mediated adaptive changes that occur in the local genital tract tissues during primary infection. These results impact our understanding of immunity to chlamydial genital infection and may provide important insight into vaccine development.
Figures






 ). The anti-chlamydial titer for immune serum and preinjection (mAbs) represents the mean titers of triplicate determinations of the pool of convalescent serum or mAb preparation before passive transfer. At 10 days postreinfection, a time in which mice had received five injections of either immune serum or mAb, blood was collected and analyzed by ELISA of anti-chlamydia Ab. Those data represent the mean titers of at least seven mice per group. Error bars are omitted from the figure, but were always <0.5 log2.
). The anti-chlamydial titer for immune serum and preinjection (mAbs) represents the mean titers of triplicate determinations of the pool of convalescent serum or mAb preparation before passive transfer. At 10 days postreinfection, a time in which mice had received five injections of either immune serum or mAb, blood was collected and analyzed by ELISA of anti-chlamydia Ab. Those data represent the mean titers of at least seven mice per group. Error bars are omitted from the figure, but were always <0.5 log2.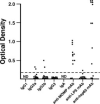
References
-
- de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–127. - PubMed
-
- Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J. Immunol. 1997;158:3344–3352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

