Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence
- PMID: 16303561
- PMCID: PMC3646525
- DOI: 10.1016/j.cub.2005.10.040
Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence
Erratum in
- Curr Biol. 2005 Dec 6;15(23):2177
Abstract
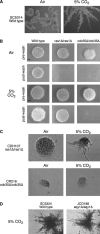
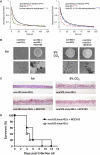
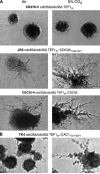
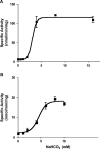
The ascomycete Candida albicans is the most common fungal pathogen in immunocompromised patients . Its ability to change morphology, from yeast to filamentous forms, in response to host environmental cues is important for virulence . Filamentation is mediated by second messengers such as cyclic adenosine 3',5'-monophosphate (cAMP) synthesized by adenylyl cyclase . The distantly related basidiomycete Cryptococcus neoformans is an encapsulated yeast that predominantly infects the central nervous system in immunocompromised patients . Similar to the morphological change in C. albicans, capsule biosynthesis in C. neoformans, a major virulence attribute, is also dependent upon adenylyl cyclase activity . Here we demonstrate that physiological concentrations of CO2/HCO3- induce filamentation in C. albicans by direct stimulation of cyclase activity. Furthermore, we show that CO2/HCO3- equilibration by carbonic anhydrase is essential for pathogenesis of C. albicans in niches where the available CO2 is limited. We also demonstrate that adenylyl cyclase from C. neoformans is sensitive to physiological concentrations of CO2/HCO3-. These data demonstrate that the link between cAMP signaling and CO2/HCO3- sensing is conserved in fungi and reveal CO2 sensing to be an important mediator of fungal pathogenesis. Novel therapeutic agents could target this pathway at several levels to control fungal infections.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

