Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury
- PMID: 16303765
- PMCID: PMC4271455
- DOI: 10.1074/jbc.M507741200
Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury
Abstract
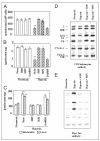
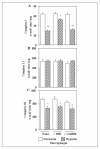
We have investigated the effects of hypoxia and myocardial ischemia/reperfusion on the structure and function of cytochrome c oxidase (CcO). Hypoxia (0.1% O(2) for 10 h) and cAMP-mediated inhibition of CcO activity were accompanied by hyperphosphorylation of subunits I, IVi1, and Vb and markedly increased reactive O(2) species production by the enzyme complex in an in vitro system that uses reduced cytochrome c as an electron donor. Both subunit phosphorylation and enzyme activity were effectively reversed by 50 nm H89 or 50 nm myristoylated peptide inhibitor (MPI), specific inhibitors of protein kinase A, but not by inhibitors of protein kinase C. In rabbit hearts subjected to global and focal ischemia, CcO activity was inhibited in a time-dependent manner and was accompanied by hyperphosphorylation as in hypoxia. Additionally, CcO activity and subunit phosphorylation in the ischemic heart were nearly completely reversed by H89 or MPI added to the perfusion medium. Hyperphosphorylation of subunits I, IVi1, and Vb was accompanied by reduced subunit contents of the immunoprecipitated CcO complex. Most interestingly, both H89 and MPI added to the perfusion medium dramatically reduced the ischemia/reperfusion injury to the myocardial tissue. Our results pointed to an exciting possibility of using CcO activity modulators for controlling myocardial injury associated with ischemia and oxidative stress conditions.
Figures







Similar articles
-
Oxidative stress induced mitochondrial protein kinase A mediates cytochrome c oxidase dysfunction.PLoS One. 2013 Oct 10;8(10):e77129. doi: 10.1371/journal.pone.0077129. eCollection 2013. PLoS One. 2013. PMID: 24130844 Free PMC article.
-
Mitochondrial LON protease-dependent degradation of cytochrome c oxidase subunits under hypoxia and myocardial ischemia.Biochim Biophys Acta Bioenerg. 2017 Jul;1858(7):519-528. doi: 10.1016/j.bbabio.2017.04.003. Epub 2017 Apr 23. Biochim Biophys Acta Bioenerg. 2017. PMID: 28442264 Free PMC article.
-
Site specific phosphorylation of cytochrome c oxidase subunits I, IVi1 and Vb in rabbit hearts subjected to ischemia/reperfusion.FEBS Lett. 2007 Apr 3;581(7):1302-10. doi: 10.1016/j.febslet.2007.02.042. Epub 2007 Feb 28. FEBS Lett. 2007. PMID: 17349628 Free PMC article.
-
Nitric oxide, cytochrome C oxidase, and the cellular response to hypoxia.Arterioscler Thromb Vasc Biol. 2010 Apr;30(4):643-7. doi: 10.1161/ATVBAHA.108.181628. Epub 2009 Aug 27. Arterioscler Thromb Vasc Biol. 2010. PMID: 19713530 Review.
-
Ischemia-reperfusion injury in the aged heart: role of mitochondria.Arch Biochem Biophys. 2003 Dec 15;420(2):287-97. doi: 10.1016/j.abb.2003.09.046. Arch Biochem Biophys. 2003. PMID: 14654068 Review.
Cited by
-
Cytochrome c Oxidase at Full Thrust: Regulation and Biological Consequences to Flying Insects.Cells. 2021 Feb 22;10(2):470. doi: 10.3390/cells10020470. Cells. 2021. PMID: 33671793 Free PMC article. Review.
-
Additive effects of mitochondrion-targeted cytochrome CYP2E1 and alcohol toxicity on cytochrome c oxidase function and stability of respirosome complexes.J Biol Chem. 2012 May 4;287(19):15284-97. doi: 10.1074/jbc.M111.314062. Epub 2012 Mar 6. J Biol Chem. 2012. PMID: 22396533 Free PMC article.
-
The Role of Mitochondrial Reactive Oxygen Species in Cardiovascular Injury and Protective Strategies.Oxid Med Cell Longev. 2016;2016:8254942. doi: 10.1155/2016/8254942. Epub 2016 Apr 21. Oxid Med Cell Longev. 2016. PMID: 27200148 Free PMC article. Review.
-
Redox homeostasis and respiratory metabolism in camels (Camelus dromedaries): comparisons with domestic goats and laboratory rats and mice.J Comp Physiol B. 2010 Nov;180(8):1121-32. doi: 10.1007/s00360-010-0482-x. Epub 2010 Jun 9. J Comp Physiol B. 2010. PMID: 20532892
-
Role of nuclear-encoded subunit Vb in the assembly and stability of cytochrome c oxidase complex: implications in mitochondrial dysfunction and ROS production.Biochem J. 2009 May 27;420(3):439-49. doi: 10.1042/BJ20090214. Biochem J. 2009. PMID: 19338496 Free PMC article.
References
-
- Chandel N, Budinger GR, Kemp RA, Schumacker PT. Am. J. Physiol. 1995;268:L918–L925. - PubMed
-
- Chandel NS, Schumacker PT. J. Appl. Physiol. 2000;88:1880–1889. - PubMed
-
- Dagsgaard C, Taylor LE, O’Brien KM, Poyton RO. J. Biol. Chem. 2001;276:7593–7601. - PubMed
-
- Merle P, Kadenbach B. Eur. J. Biochem. 1982;125:239–244. - PubMed
-
- Poyton RO, Dagsgaard CJ. Adv. Exp. Med. Biol. 2000;475:177–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

