Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis
- PMID: 16303961
- PMCID: PMC1364483
- DOI: 10.1167/iovs.05-0843
Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis
Abstract
Purpose: The role of oxygen in the formation of lens high-molecular-weight (HMW) protein aggregates during the development of human nuclear cataract is not well understood. The purpose of this study was to investigate lens crystallin aggregate formation in hyperbaric oxygen (HBO)-treated guinea pigs by using in vivo and in vitro
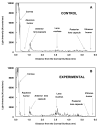
Methods: methods. Guinea pigs were treated three times weekly for 7 months with HBO, and lens crystallin aggregation was investigated in vivo with the use of dynamic light-scattering (DLS) and in vitro by HPLC analysis of water-insoluble (WI) proteins. DLS measurements were made every 0.1 mm across the 4.5- to 5.0-mm optical axis of the guinea pig lens.
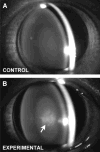
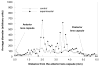
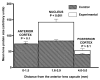
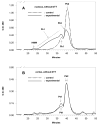
Results: The average apparent diameter of proteins in the nucleus (the central region) of lenses of HBO-treated animals was nearly twice that of the control animals (P < 0.001). Size distribution analysis conducted at one selected point in the nucleus and cortex (the outer periphery of the lens) after dividing the proteins into small-diameter and large-diameter groups, showed in the O2-treated nucleus a threefold increase in intensity (P < 0.001) and a doubling in apparent size (P = 0.03) of large-diameter aggregate proteins, compared with the same control group. No significant changes in apparent protein diameter were detected in the O2-treated cortex, compared with the control. The average diameter of protein aggregates at the single selected location in the O2-treated nucleus was estimated to be 150 nm, a size capable of scattering light and similar to the size of aggregates found in human nuclear cataracts. HPLC analysis indicated that one half of the experimental nuclear WI protein fraction (that had been dissolved in guanidine) consisted of disulfide cross-linked 150- to 1000-kDa aggregates, not present in the control. HPLC-isolated aggregates contained alphaA-, beta-, gamma-, and zeta-crystallins, but not alphaB-crystallin, which is devoid of -SH groups and thus does not participate in disulfide cross-linking. All zeta-crystallin present in the nuclear WI fraction appeared to be there as a result of disulfide cross-linking.
Conclusions: The results indicate that molecular oxygen in vivo can induce the cross-linking of guinea pig lens nuclear crystallins into large disulfide-bonded aggregates capable of scattering light. A similar process may be involved in the formation of human nuclear cataract.
Figures

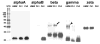
Similar articles
-
Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen.Exp Eye Res. 1995 Mar;60(3):219-35. doi: 10.1016/s0014-4835(05)80105-8. Exp Eye Res. 1995. PMID: 7789403
-
The effects of hyperbaric oxygen on the crystallins of cultured rabbit lenses: a possible catalytic role for copper.Exp Eye Res. 2000 Oct;71(4):371-83. doi: 10.1006/exer.2000.0887. Exp Eye Res. 2000. PMID: 10995558
-
Shotgun proteomic analysis of S-thiolation sites of guinea pig lens nuclear crystallins following oxidative stress in vivo.Mol Vis. 2013;19:267-80. Epub 2013 Feb 3. Mol Vis. 2013. PMID: 23401655 Free PMC article.
-
Genetics of crystallins: cataract and beyond.Exp Eye Res. 2009 Feb;88(2):173-89. doi: 10.1016/j.exer.2008.10.011. Epub 2008 Nov 1. Exp Eye Res. 2009. PMID: 19007775 Review.
-
Hyperbaric oxygen as a model of lens aging in the bovine lens: The effects on lens biochemistry, physiology and optics.Exp Eye Res. 2021 Nov;212:108790. doi: 10.1016/j.exer.2021.108790. Epub 2021 Oct 11. Exp Eye Res. 2021. PMID: 34648773 Review.
Cited by
-
Investigation of scleral thermal injuries caused by ultrasonic pars plana phacoemulsification and aspiration using pig eyes.Int Ophthalmol. 2019 Sep;39(9):2015-2021. doi: 10.1007/s10792-018-1036-6. Epub 2018 Oct 23. Int Ophthalmol. 2019. PMID: 30353259
-
Enzyme-induced posterior vitreous detachment in the rat produces increased lens nuclear pO2 levels.Exp Eye Res. 2009 Feb;88(2):286-92. doi: 10.1016/j.exer.2008.09.003. Epub 2008 Sep 18. Exp Eye Res. 2009. PMID: 18835558 Free PMC article.
-
UV filters in the lens of the thirteen lined ground squirrel (Spermophilus tridecemlineatus).Exp Eye Res. 2006 Apr;82(4):730-7. doi: 10.1016/j.exer.2005.09.014. Epub 2005 Nov 17. Exp Eye Res. 2006. PMID: 16297909 Free PMC article.
-
Deletion of mouse MsrA results in HBO-induced cataract: MsrA repairs mitochondrial cytochrome c.Mol Vis. 2009 May 15;15:985-99. Mol Vis. 2009. PMID: 19461988 Free PMC article.
-
A Class I UV-blocking (senofilcon A) soft contact lens prevents UVA-induced yellow fluorescence and NADH loss in the rabbit lens nucleus in vivo.Exp Eye Res. 2012 Sep;102:17-27. doi: 10.1016/j.exer.2012.06.007. Epub 2012 Jul 2. Exp Eye Res. 2012. PMID: 22766154 Free PMC article.
References
-
- The Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. - PubMed
-
- Adamsons I, Munoz B, Enger C, Taylor HR. Prevalence of lens opacities in surgical and general populations. Arch Ophthalmol. 1991;109:993–997. - PubMed
-
- Congdon NC, Taylor HR. Age-related cataract. In: Weale RA, West SK, Johnson GJ, Minassian DC, eds. The Epidemiology of Eye Disease. London: Arnold; 2003:106–107.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

