Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells
- PMID: 16304044
- PMCID: PMC1895390
- DOI: 10.1182/blood-2005-09-3541
Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells
Abstract
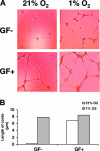
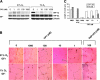
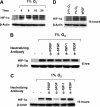
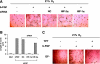
Hypoxia is a major pathophysiological condition for the induction of angiogenesis, which is a crucial aspect of growth in solid tumors. In mammalian cells, the transcriptional response to oxygen deprivation is largely mediated by hypoxia-inducible factor 1 (HIF-1), a heterodimer composed of HIF-1alpha and HIF-1beta subunits. However, the response of endothelial cells to hypoxia and the specific involvement of HIF-alpha subunits in this process are still poorly understood. We show that human umbilical vein endothelial cells (HUVECs) cultured in the absence of growth factors survive and form tubelike structures when cultured under hypoxic, but not normoxic, conditions. HUVECs expressed both HIF-1alpha and HIF-2alpha when cultured under hypoxic conditions. Transfection of HIF-1alpha, but not HIF-2alpha, siRNA to HUVECs completely abrogated hypoxic induction of cords. Neutralizing antibodies to bFGF, but not IGF-1, VEGF, or PDGF-BB, blocked survival and sprouting of HUVECs under hypoxic conditions, suggesting the existence of an autocrine loop induced by low oxygen levels. Notably, bFGF-dependent induction of cord formation under normoxic conditions required HIF-1alpha activity, which was also essential for hypoxic induction of bFGF mRNA and protein expression. These results uncover the existence of an HIF-1alpha-bFGF amplification pathway that mediates survival and sprouting of endothelial cells under hypoxic conditions.
Figures




References
-
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87: 1153-1155. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407: 249-257. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86: 353-364. - PubMed
-
- Acker T, Plate KH. Role of hypoxia in tumor angiogenesis-molecular and cellular angiogenic crosstalk. Cell Tissue Res. 2003;314: 145-155. - PubMed
-
- Bicknell R, Harris AL. Novel angiogenic signaling pathways and vascular targets. Annu Rev Pharmacol Toxicol. 2004;44: 219-238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

