Chemokine and chemokine receptor expression during colony stimulating factor-1-induced osteoclast differentiation in the toothless osteopetrotic rat: a key role for CCL9 (MIP-1gamma) in osteoclastogenesis in vivo and in vitro
- PMID: 16304045
- PMCID: PMC1895722
- DOI: 10.1182/blood-2005-08-3365
Chemokine and chemokine receptor expression during colony stimulating factor-1-induced osteoclast differentiation in the toothless osteopetrotic rat: a key role for CCL9 (MIP-1gamma) in osteoclastogenesis in vivo and in vitro
Abstract
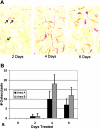
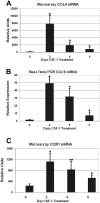
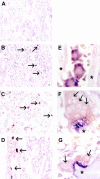
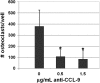
Osteoclasts differentiate from hematopoietic precursors under systemic and local controls. Chemokines and receptors direct leukocyte traffic throughout the body and may help regulate site-specific bone resorption. We investigated bone gene expression in vivo during rapid osteoclast differentiation induced by colony-stimulating factor 1 (CSF-1) in Csf1-null toothless (tl/tl) rats. Long-bone RNA from CSF-1-treated tl/tl rats was analyzed by high-density microarray over a time course. TRAP (tartrate-resistant acid phosphatase)-positive osteoclasts appeared on day 2, peaked on day 4, and decreased slightly on day 6, as marrow space was expanding. TRAP and cathepsin K mRNA paralleled the cell counts. We examined all chemokine and receptor mRNAs on the arrays. CCL9 was strongly induced and peaked on day 2, as did its receptor, CCR1, and regulatory receptors c-Fms (CSF-1 receptor) and RANK (receptor activator of nuclear factor kappaB). Other chemokines and receptors showed little or no significant changes. In situ hybridization and immunohistochemistry revealed CCL9 in small, immature osteoclasts on day 2 and in mature cells at later times. Anti-CCL9 antibody inhibited osteoclast differentiation in culture and significantly suppressed the osteoclast response in CSF-1-treated tl/tl rats. While various chemokines have been implicated in osteoclastogenesis in vitro, this first systematic analysis of chemokines and receptors during osteoclast differentiation in vivo highlights the key role of CCL9 in this process.
Figures

Similar articles
-
CCL9/MIP-1gamma and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts.J Cell Biochem. 2002;87(4):386-93. doi: 10.1002/jcb.10319. J Cell Biochem. 2002. PMID: 12397598
-
MIP-1 gamma promotes receptor-activator-of-NF-kappa-B-ligand-induced osteoclast formation and survival.J Immunol. 2004 Aug 1;173(3):2084-90. doi: 10.4049/jimmunol.173.3.2084. J Immunol. 2004. PMID: 15265944
-
The effects of colony-stimulating factor-1 (CSF-1) on the development of osteoclasts and their expression of tartrate-resistant acid phosphatase (TRAP) in toothless (tl-osteopetrotic) rats.Crit Rev Eukaryot Gene Expr. 2003;13(2-4):117-32. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.60. Crit Rev Eukaryot Gene Expr. 2003. PMID: 14696961
-
Regulation of osteoclast differentiation by fibroblast growth factor 2: stimulation of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor expression in osteoblasts and inhibition of macrophage colony-stimulating factor function in osteoclast precursors.J Bone Miner Res. 2001 Nov;16(11):2074-81. doi: 10.1359/jbmr.2001.16.11.2074. J Bone Miner Res. 2001. PMID: 11697804
-
Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis.J Biol Chem. 2006 Aug 18;281(33):23598-605. doi: 10.1074/jbc.M602191200. Epub 2006 Jun 19. J Biol Chem. 2006. PMID: 16787916
Cited by
-
Oral inflammatory diseases and systemic inflammation: role of the macrophage.Front Immunol. 2012 May 16;3:118. doi: 10.3389/fimmu.2012.00118. eCollection 2012. Front Immunol. 2012. PMID: 22623923 Free PMC article.
-
The role of biomineralization in disorders of skeletal development and tooth formation.Nat Rev Endocrinol. 2021 Jun;17(6):336-349. doi: 10.1038/s41574-021-00488-z. Epub 2021 May 4. Nat Rev Endocrinol. 2021. PMID: 33948016 Review.
-
Deficiency of chemokine receptor CCR1 causes osteopenia due to impaired functions of osteoclasts and osteoblasts.J Biol Chem. 2010 Sep 10;285(37):28826-37. doi: 10.1074/jbc.M109.099424. Epub 2010 Jun 22. J Biol Chem. 2010. PMID: 20571024 Free PMC article.
-
Monocyte Chemotactic Protein-1 (MCP1) Accumulation in Human Osteoclast Precursor Cultures.Life (Basel). 2022 May 26;12(6):789. doi: 10.3390/life12060789. Life (Basel). 2022. PMID: 35743820 Free PMC article.
-
CCL4 enhances preosteoclast migration and its receptor CCR5 downregulation by RANKL promotes osteoclastogenesis.Cell Death Dis. 2018 May 1;9(5):495. doi: 10.1038/s41419-018-0562-5. Cell Death Dis. 2018. PMID: 29717113 Free PMC article.
References
-
- Marks SC, Jr., Odgren PR. The structure and development of the skeleton. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology, Vol. 1 (2nd ed). New York, NY: Academic Press; 2002: 3-15.
-
- Whyte MP. Sclerosing bone disorders. In: Fauvus MJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (5th ed). Washington, D.C.: American Society for Bone and Mineral Research; 2003: 449-466.
-
- de Vernejoul MC, Benichou O. Human osteopetrosis and other sclerosing disorders: recent genetic developments. Calcif Tissue Int. 2001;69: 1-6. - PubMed
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423: 337-342. - PubMed
-
- Moutier R, Toyama K, Cotton WR, Gaines JF. Three recessive genes for congenital osteopetrosis in the Norway rat. J Heredity. 1976;67: 189-190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

