Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies
- PMID: 16304155
- PMCID: PMC1315926
- DOI: 10.1128/AAC.49.12.4934-4941.2005
Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies
Abstract
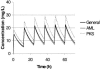
This study determines vancomycin (VAN) population pharmacokinetics (PK) in adult patients with hematological malignancies. VAN serum concentration data (n = 1,004) from therapeutic drug monitoring were collected retrospectively from 215 patients. A one-compartment PK model was selected. VAN pharmacokinetics population parameters were generated using the NONMEM program. A graphic approach and stepwise generalized additive modeling were used to elucidate the preliminary relationships between PK parameters and clinical covariates analyzed. Covariate selection revealed that total body weight (TBW) affected V, whereas renal function, estimated by creatinine clearance, and a diagnosis of acute myeloblastic leukemia (AML) influenced VAN clearance. We propose one general and two AML-specific models. The former was defined by CL (liters/h) = 1.08 x CL(CR(Cockcroft and Gault)) (liters/h); CV(CL) = 28.16% and V (liters) = 0.98 x TBW; CV(V) =37.15%. AML models confirmed this structure but with a higher clearance coefficient (1.17). The a priori performance of the models was evaluated in another 59 patients, and clinical suitability was confirmed. The models were fairly accurate, with more than 33% of the measured concentrations being within +/-20% of the predicted value. This therapeutic precision is twofold higher than that of a non-customized population model (16.1%). The corresponding standardized prediction errors included zero and a standard deviation close to unity. The models could be used to estimate appropriate VAN dosage guidelines, which are not clearly defined for this high-risk population. Their simple structure should allow easy implementation in clinical software and application in dosage individualization using the Bayesian approach.
Figures


References
-
- Abbott Laboratories. 1991. AbbottBase Pharmacokinetics System. Abbott Laboratories, Diagnostic Division, Abbott Park, Ill.
-
- Akaike, H. 1979. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 66:237-242.
-
- Barret, J. S. 2002. Population pharmacokinetics, p. 315-356. In R. D. Schoenwald (ed.), Pharmacokinetics in drug discovery and development. CRC Press, Boca Raton, Fla.
-
- Beal, S. L., and L. B. Sheiner. 1989. NONMEM user's guides. NONMEM project group. University of California, San Francisco.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

