Absolute bioavailability and intracellular pharmacokinetics of azithromycin in patients with cystic fibrosis
- PMID: 16304166
- PMCID: PMC1315964
- DOI: 10.1128/AAC.49.12.5013-5017.2005
Absolute bioavailability and intracellular pharmacokinetics of azithromycin in patients with cystic fibrosis
Abstract
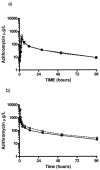
Chronic pulmonary infections with Pseudomonas aeruginosa are the primary cause of morbidity and mortality in patients with cystic fibrosis (CF). The macrolide antibiotics exhibit immunomodulatory and antivirulence activity. Clinical trials with azithromycin in CF have demonstrated significant improvements in pulmonary function and decreased hospitalizations. The purpose of this study was to compare the pharmacokinetics (PK) of azithromycin in patients with CF and controls. The study was conducted as an open-label, parallel, two-period crossover study involving 12 healthy volunteers and 12 patients with CF. Period 1 examined the serum PK following a single oral and intravenous dose, while period 2 examined the intracellular PK following multiple-dose oral administration. CF subjects differed significantly from controls based on weight (53.1 versus 71.0 kg; P < 0.01) and body mass index (19.7 versus 23.2; P < 0.01), respectively. Ninety-two percent of CF patients were pancreatic insufficient and were receiving pancreatic enzymes. The rate (time to reach maximum serum drug concentration, 3.0 versus 3.0 h; P = 0.78) and extent of absorption (absolute bioavailability, 34.2 versus 42.8%; P = 0.37) were similar in patients with CF and controls, respectively. Distribution to the tissues (rate of drug transfer from the central to the peripheral compartment, 1.22 versus 0.759 h(-1); P = 0.03) and elimination (rate of elimination from the central compartment, 0.693 versus 0.492 h(-1); P < 0.01) were more rapid in the healthy volunteers than in the CF subjects, respectively. Mononuclear cell concentrations (15.2 +/- 6.0 mg/liter) far exceeded the maximum serum drug concentration ( approximately 50-fold), demonstrating significant intracellular accumulation. These results indicate no alteration in dosage of azithromycin is necessary in patients with CF taking pancreatic enzymes.
Figures

References
-
- Amsden, G. W., and C. L. Gray. 2001. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single dose or over a 3 day period in healthy volunteers. J. Antimicrob. Chemother. 47:61-66. - PubMed
-
- Bonfield, T. L., M. W. Konstan, and M. Berger. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152:2111-2118. - PubMed
-
- Bouquet, J., M. Sinaasappel, and H. Neijens. 1988. Malabsorption in cystic fibrosis: mechanisms and treatment. J. Pediatr. Gastroenterol. Nutr. 7(Suppl. 1):S30-S35. - PubMed
-
- Costerton, J. 2001. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol. 9:50-52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

