In vitro activity of linezolid against key gram-positive organisms isolated in the united states: results of the LEADER 2004 surveillance program
- PMID: 16304168
- PMCID: PMC1315934
- DOI: 10.1128/AAC.49.12.5024-5032.2005
In vitro activity of linezolid against key gram-positive organisms isolated in the united states: results of the LEADER 2004 surveillance program
Abstract
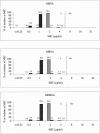
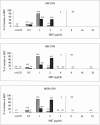
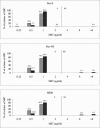
Since the approval of linezolid in 2000, sporadic reports of resistance have been given and a greater understanding of the underlying mechanisms of resistance has been gained. However, since these developments, an updated status of the in vitro activity of linezolid against gram-positive organisms from the United States has not been reported. The LEADER 2004 surveillance initiative was undertaken to obtain current and representative data on the activity of linezolid against key species, including isolates with significant resistance phenotypes. Organisms were isolated during 2004 and included 2,872 Staphylococcus aureus, 496 coagulase-negative staphylococcus (CNS), 428 Enterococcus faecalis, 196 Enterococcus faecium, and 422 Streptococcus pneumoniae isolates. All S. aureus isolates (54.2% oxacillin resistant) were susceptible to linezolid (MIC90 = 2 microg/ml); MIC distributions were consistent, regardless of oxacillin or multidrug resistance status. For CNS, one nonsusceptible isolate was encountered (Staphylococcus epidermidis; MIC = 32 microg/ml), but overall, the MIC(90) (1 microg/ml) was lower than that obtained with S. aureus. For E. faecalis and E. faecium, 99.5% and 96.4% of isolates, respectively, were linezolid susceptible. Both species had an MIC90 of 2 microg/ml, and MIC distributions did not vary with the vancomycin susceptibility status of the populations analyzed. Linezolid nonsusceptibility was not encountered among the S. pneumoniae isolates. These findings indicate that linezolid nonsusceptibility has remained rare among staphylococci and uncommon and sporadic among enterococci. Nonetheless, careful and ongoing monitoring of the in vitro effectiveness of linezolid will be needed so that any changes to the current status may be detected as soon as possible.
Figures

References
-
- Ballow, C. H., R. N. Jones, D. J. Biedenbach, and the North American ZAPS Research Group. 2002. A multicenter evaluation of linezolid antimicrobial activity in North America. Diagn. Microbiol. Infect. Dis. 43:75-83. - PubMed
-
- Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing, 15th informational supplement, vol. 25, no. 1. M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
-
- Diekema, D. J., B. J. BootsMiller, T. E. Vaughn, R. F. Woolson, J. W. Yankey, E. J. Ernst, S. D. Flach, M. M. Ward, C. L. J. Franciscus, M. A. Pfaller, and B. N. Doebbeling. 2004. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin. Infect. Dis. 38:78-85. - PubMed
-
- Farrell, D. J., I. Morrissey, S. Bakker, S. Buckridge, and D. Felmingham. 2004. In vitro activities of telithromycin, linezolid, and quinupristin-dalfopristin against Streptococcus pneumoniae with macrolide resistance due to ribosomal mutations. Antimicrob. Agents Chemother. 48:3169-3171. - PMC - PubMed
-
- Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

