Efficacy of caspofungin against Aspergillus terreus
- PMID: 16304185
- PMCID: PMC1315968
- DOI: 10.1128/AAC.49.12.5133-5135.2005
Efficacy of caspofungin against Aspergillus terreus
Abstract
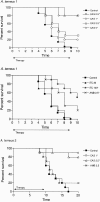
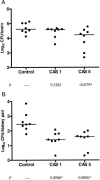
We investigated the in vitro and in vivo activities of caspofungin against Aspergillus terreus. The drug increased survival and reduced tissue fungal burden in neutropenic mice. Therefore, our data support the role of caspofungin in treating systemic infections due to this emerging pathogen.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

