Sulphation of o-desmethylnaproxen and related compounds by human cytosolic sulfotransferases
- PMID: 16305588
- PMCID: PMC1884888
- DOI: 10.1111/j.1365-2125.2005.02506.x
Sulphation of o-desmethylnaproxen and related compounds by human cytosolic sulfotransferases
Abstract
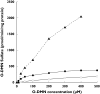
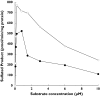
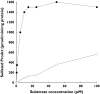
Naproxen is a nonsteroidal anti-inflammatory drug widely used as an analgesic and anti-inflammatory agent. The conjugated forms of naproxen and O-DMN, its demethylated metabolite, account for 66-92% of naproxen found in human urine. In this study, O-DMN and structurally related compounds were tested as substrates for seven isoforms of human cytosolic sulfotransferase (SULT). The SULT2 or hydroxysteroid SULT isoforms, SULT2A1 and SULT2B1b, did not show reactivity with any of the compounds. All five SULT1 isoforms were active although there was variability between SULT isoforms and compounds assayed. O-DMN was sulphated by SULT1A1, SULT1B1 and SULT1E1. All five SULT1 isoforms were capable of conjugating both alpha-naphthol and beta-naphthol. Apparent Km values for O-DMN sulphation were significantly higher than the values for either alpha-naphthol or beta-naphthol. SULTs 1A1, 1B1 and 1E1 had Kms for O-DMN sulphation of 84 microM, 690 microM and 341 microM, respectively. These Km values were 40-1150-fold higher than the Km values for alpha- and beta-naphthol. The role of the side-chain in O-DMN sulphation was studied using a series of structurally related beta-naphthol compounds as substrates for SULT1A1 and SULT1E1. The presence of lipophilic groups increased affinity for both SULT isoforms whereas inclusion of a carboxyl group inhibited activity. These studies indicate that O-DMN is sulphated by SULT1A1, B1 and 1E1. Because of the high concentrations of SULT1A1 expression in human liver and intestines and its higher affinity for O-DMN sulphation, SULT1A1 may have a role in the first pass metabolism of O-DMN.
Figures
Similar articles
-
Effects of the human SULT1A1 polymorphisms on the sulfation of acetaminophen,O-desmethylnaproxen, and tapentadol.Pharmacol Rep. 2019 Apr;71(2):257-265. doi: 10.1016/j.pharep.2018.12.001. Epub 2018 Dec 10. Pharmacol Rep. 2019. PMID: 30822619 Free PMC article.
-
A reappraisal of the 6-O-desmethylnaproxen-sulfating activity of the human cytosolic sulfotransferases.Can J Physiol Pharmacol. 2017 Jun;95(6):647-651. doi: 10.1139/cjpp-2016-0403. Epub 2017 Jan 22. Can J Physiol Pharmacol. 2017. PMID: 28177672
-
Sulfation of apomorphine by human sulfotransferases: evidence of a major role for the polymorphic phenol sulfotransferase, SULT1A1.Xenobiotica. 2003 Nov;33(11):1139-48. doi: 10.1080/00498250310001609192. Xenobiotica. 2003. PMID: 14660177
-
Structure, function and polymorphism of human cytosolic sulfotransferases.Curr Drug Metab. 2008 Feb;9(2):99-105. doi: 10.2174/138920008783571819. Curr Drug Metab. 2008. PMID: 18288952 Review.
-
Regulation of sulfotransferase mRNA expression in male and female rats of various ages.Chem Biol Interact. 1998 Feb 20;109(1-3):299-313. doi: 10.1016/s0009-2797(97)00141-5. Chem Biol Interact. 1998. PMID: 9566754 Review.
Cited by
-
On the Molecular Basis Underlying the Metabolism of Tapentadol Through Sulfation.Eur J Drug Metab Pharmacokinet. 2017 Oct;42(5):793-800. doi: 10.1007/s13318-016-0392-8. Eur J Drug Metab Pharmacokinet. 2017. PMID: 28070880
-
Effects of the human SULT1A1 polymorphisms on the sulfation of acetaminophen,O-desmethylnaproxen, and tapentadol.Pharmacol Rep. 2019 Apr;71(2):257-265. doi: 10.1016/j.pharep.2018.12.001. Epub 2018 Dec 10. Pharmacol Rep. 2019. PMID: 30822619 Free PMC article.
-
Sulforaphane Alters β-Naphthoflavone-Induced Changes in Activity and Expression of Drug-Metabolizing Enzymes in Rat Hepatocytes.Molecules. 2017 Nov 16;22(11):1983. doi: 10.3390/molecules22111983. Molecules. 2017. PMID: 29144397 Free PMC article.
-
Specific estrogen sulfotransferase (SULT1E1) substrates and molecular imaging probe candidates.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6222-7. doi: 10.1073/pnas.0914904107. Epub 2010 Mar 19. Proc Natl Acad Sci U S A. 2010. PMID: 20304798 Free PMC article.
-
Transcriptomic profiling of hepatic tissues for drug metabolism genes in nonalcoholic fatty liver disease: A study of human and animals.Front Endocrinol (Lausanne). 2023 Jan 4;13:1034494. doi: 10.3389/fendo.2022.1034494. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36686439 Free PMC article.
References
-
- Segre EJ. Naproxen metabolism in man. J Clin Pharmacol. 1975;15:316–23. - PubMed
-
- Davies NM, Anderson KE. Clinical pharmacokinetics of naproxen. Clin Pharmacokinet. 1997;32:268–93. - PubMed
-
- Vree TB, van den Biggelaar-Martea M, Verwey-van Wissen CP. Determination of naproxen and its metabolite O-desmethylnaproxen with their acyl glucuronides in human plasma and urine by means of direct gradient high-performance liquid chromatography. J Chromatogr. 1992;578:239–49. - PubMed
-
- Kiang CH, Lee C, Kushinsky S. Isolation and identification of 6-desmethylnaproxen sulfate as a new metabolite of naproxen in human plasma. Drug Metab Dispos. 1989;17:43–8. - PubMed
-
- Falany CN. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11:206–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

