Dramatic change in prescribing of hormone replacement therapy in The Netherlands after publication of the Million Women Study: a follow-up study
- PMID: 16305589
- PMCID: PMC1884890
- DOI: 10.1111/j.1365-2125.2005.02502.x
Dramatic change in prescribing of hormone replacement therapy in The Netherlands after publication of the Million Women Study: a follow-up study
Abstract
Aims: To estimate the diffusion of new safety information concerning postmenopausal hormonal replacement therapy (HRT) into prescribing practice in The Netherlands and to assess the impact of revised guidelines on the long-term treatment of HRT.
Design: Cross-sectional study.
Setting: Community pharmacy dispensing data from a population of approximately 450,000 patients in the northern and eastern part of The Netherlands.
Population: Women aged 45-69 years to whom at least one HRT prescription was dispensed between 1 January 2000 and 1 January 2004.
Main outcome measures: Annual and quarter prevalences of HRT and the proportion of new HRT users, switchers and continuous HRT users per quarter.
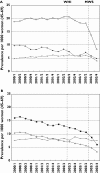
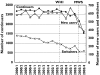
Results: The prevalence of HRT prescribing decreased significantly from 107/1000 [95% confidence interval (CI) 104, 110] in 2000 to 87/1000 (95% CI 84, 89) in 2003. The decreasing prevalence was especially evident among the younger age groups and was most pronounced among users of oestrogen/progestagen combinations. The publication of the Women Health Initiative Study (WHI) was followed by a modest decrease in prescribing of HRT, whereas prescribing of HRT declined dramatically after publication of the Million Women Study (MWS) in August 2003. Among the continuous HRT users in the 4th quarter of 2002, 55% used HRT longer than 3 years. This percentage was 53 in the 4th quarter of 2003.
Conclusions: In contrast to the release of the WHI study results, publication of the MWS was followed by a dramatic fall in prescribing of HRT in The Netherlands. Despite the new recommendation that long-term HRT use should be discouraged, the proportion of long-term users did not change after the publication of the MWS.
Figures

 )
)
References
-
- Writing group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results form the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. - PubMed
-
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women's Health Initiative Memory study: a randomized controlled trial. JAMA. 2003;289:2651–62. - PubMed
-
- Writing group for the Women's Health Initiative Investigators. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701–12. - PubMed
-
- Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. - PubMed
-
- Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–61. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

