Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis
- PMID: 16306044
- PMCID: PMC4376329
- DOI: 10.1074/jbc.M509866200
Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis
Abstract
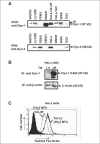
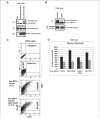
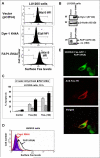
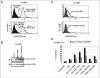
Human melanoma is the most aggressive form of skin cancer and is extremely resistant to radiation and chemotherapy. One of the critical parameters of this resistance is down-regulation of Fas (CD95) cell-surface expression. Using TIG3 normal human fibroblasts and human melanoma cell lines, we investigated transcriptional regulation of FAP-1, a regulator of Fas translocation in the cell. Protein-tyrosine phosphatase FAP-1 (PTPN13, PTP-BAS) interacts with human Fas protein and prevents its export from the cytoplasm to the cell surface. In contrast, dynamin-2 facilitates Fas protein translocation from the Golgi apparatus via the trans-Golgi network to the cell surface. Suppression of dynamin functions by dominant negative dynamin K44A blocks Fas export, whereas the down-regulation of FAP-1 expression by specific RNA interference restores Fas export (a phenomenon that could still be down-regulated in the presence of dominant-negative dynamin). Based on the FAP-1- and dynamin-dependent regulation of Fas translocation, we have created human melanoma lines with different levels of surface expression of Fas. Treatment of these melanoma lines with soluble Fas ligand resulted in programmed cell death that was proportional to the pre-existing levels of surface Fas. Taking into consideration the well known observations that FAP-1 expression is often up-regulated in metastatic tumors, we have established a causal connection between high basal NF-kappaB transcription factor activity (which is a hallmark of many types of metastatic tumors) and NF-kappaB-dependent transcriptional regulation of FAP-1 gene expression that finally restricts Fas protein trafficking, thereby, facilitating the survival of cancer cells.
Figures





Similar articles
-
FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface.Mol Cell Biol. 2003 May;23(10):3623-35. doi: 10.1128/MCB.23.10.3623-3635.2003. Mol Cell Biol. 2003. PMID: 12724420 Free PMC article.
-
FAP-1 in pancreatic cancer cells: functional and mechanistic studies on its inhibitory role in CD95-mediated apoptosis.J Cell Sci. 2001 Aug;114(Pt 15):2735-46. doi: 10.1242/jcs.114.15.2735. J Cell Sci. 2001. PMID: 11683408
-
No evidence for involvement of mouse protein-tyrosine phosphatase-BAS-like Fas-associated phosphatase-1 in Fas-mediated apoptosis.J Biol Chem. 1997 Nov 28;272(48):30215-20. doi: 10.1074/jbc.272.48.30215. J Biol Chem. 1997. PMID: 9374505
-
[Development of anti cancer drugs targeted on Fas-mediated apoptosis signal].Gan To Kagaku Ryoho. 1997 Jan;24(2):222-8. Gan To Kagaku Ryoho. 1997. PMID: 9030235 Review. Japanese.
-
Soluble HLA class I molecules/CD8 ligation trigger apoptosis of CD8+ cells by Fas/Fas-ligand interaction.ScientificWorldJournal. 2002 Feb 12;2:421-3. doi: 10.1100/tsw.2002.122. ScientificWorldJournal. 2002. PMID: 12806026 Free PMC article. Review.
Cited by
-
The Effect of Caffeic Acid on Spermatogonial Stem Cell-type A Cryopreservation.Rep Biochem Mol Biol. 2018 Oct;7(1):85-93. Rep Biochem Mol Biol. 2018. PMID: 30324122 Free PMC article.
-
NF-kappaB activation in melanoma.Pigment Cell Res. 2006 Apr;19(2):112-24. doi: 10.1111/j.1600-0749.2006.00304.x. Pigment Cell Res. 2006. PMID: 16524427 Free PMC article. Review.
-
Inhibition of SREBP1 sensitizes cells to death ligands.Oncotarget. 2011 Mar;2(3):186-96. doi: 10.18632/oncotarget.239. Oncotarget. 2011. PMID: 21406729 Free PMC article.
-
A review of Dynamin 2 involvement in cancers highlights a promising therapeutic target.J Exp Clin Cancer Res. 2021 Jul 22;40(1):238. doi: 10.1186/s13046-021-02045-y. J Exp Clin Cancer Res. 2021. PMID: 34294140 Free PMC article. Review.
-
Polyubiquitination of p62/SQSTM1 is a prerequisite for Fas/CD95 aggregation to promote caspase-dependent apoptosis in cadmium-exposed mouse monocyte RAW264.7 cells.Sci Rep. 2019 Aug 22;9(1):12240. doi: 10.1038/s41598-019-48684-2. Sci Rep. 2019. PMID: 31439879 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

