Antagonistic functions of tetradecanoyl phorbol acetate-inducible-sequence 11b and HuR in the hormonal regulation of vascular endothelial growth factor messenger ribonucleic acid stability by adrenocorticotropin
- PMID: 16306087
- PMCID: PMC2214857
- DOI: 10.1210/me.2005-0121
Antagonistic functions of tetradecanoyl phorbol acetate-inducible-sequence 11b and HuR in the hormonal regulation of vascular endothelial growth factor messenger ribonucleic acid stability by adrenocorticotropin
Abstract
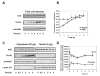
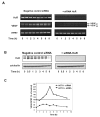
Expression of vascular endothelial growth factor (VEGF), an endothelial cell-specific mitogen and a potent angiogenic factor, is up-regulated by a variety of factors including hypoxia, growth factors, and hormones. In the adrenal cortex, regulation of VEGF expression by the pituitary hormone ACTH ensures the maintenance of the organ vasculature. We have previously shown that ACTH evokes a rapid and transient increase in VEGF mRNA levels in primary adrenocortical cells through transcription-independent mechanisms. We further demonstrated that the zinc finger RNA-binding protein Tis11b (tetradecanoyl phorbol acetate-inducible-sequence 11b) destabilizes VEGF mRNA through its 3'-untranslated region (3'-UTR) and that Tis11b is involved in the decay phase of ACTH-induced VEGF mRNA expression. In the present study, we attempted to determine the mechanisms underlying ACTH-elicited increase in VEGF mRNA levels in adrenocortical cells. We show that ACTH triggers an increase in the levels of the mRNA-stabilizing protein HuR in the cytoplasm and a concomitant decrease in the levels of HuR in the nucleus. This process is accompanied by an increased association of HuR with the nucleocytoplasmic shuttling protein pp32, indicating that ACTH induces HuR translocation from the nuclear to the cytoplasmic compartment. Leptomycin B, a specific inhibitor of CRM1-dependent nuclear export of pp32, significantly reduced ACTH-induced VEGF mRNA levels. Furthermore, RNA interference-mediated depletion of HuR in adrenocortical cells abrogated ACTH-induced VEGF mRNA expression. Finally, we show that Tis11b and HuR exert antagonistic effects on VEGF 3'-UTR in vitro. Although both proteins could bind simultaneously on VEGF 3'-UTR, Tis11b markedly decreases HuR-binding to this RNA sequence. Altogether, these results suggest that the RNA-stabilizing protein HuR is instrumental to ACTH-induced expression of VEGF mRNA and that the nuclear export of HuR is a rate-limiting step in this process. HuR appears to transiently stabilize VEGF transcripts after ACTH stimulation of adrenocortical cells, and Tis11b appears to subsequently trigger their degradation.
Figures





References
-
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. - PubMed
-
- Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. - PubMed
-
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
-
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

