Role of salicylic acid and fatty acid desaturation pathways in ssi2-mediated signaling
- PMID: 16306139
- PMCID: PMC1310554
- DOI: 10.1104/pp.105.071662
Role of salicylic acid and fatty acid desaturation pathways in ssi2-mediated signaling
Abstract
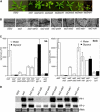
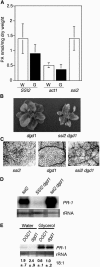
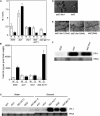
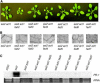
Stearoyl-acyl carrier protein desaturase-mediated conversion of stearic acid to oleic acid (18:1) is the key step that regulates the levels of unsaturated fatty acids (FAs) in cells. Our previous work with the Arabidopsis (Arabidopsis thaliana) ssi2/fab2 mutant and its suppressors demonstrated that a balance between glycerol-3-phosphate (G3P) and 18:1 levels is critical for the regulation of salicylic acid (SA)- and jasmonic acid-mediated defense signaling in the plant. In this study, we have evaluated the role of various genes that have an impact on SA, resistance gene-mediated, or FA desaturation (FAD) pathways on ssi2-mediated signaling. We show that ssi2-triggered resistance is dependent on EDS1, PAD4, EDS5, SID2, and FAD7 FAD8 genes. However, ssi2-triggered defects in the jasmonic acid pathway, morphology, and cell death phenotypes are independent of the EDS1, EDS5, PAD4, NDR1, SID2, FAD3, FAD4, FAD5, DGD1, FAD7, and FAD7 FAD8 genes. Furthermore, the act1-mediated rescue of ssi2 phenotypes is also independent of the FAD2, FAD3, FAD4, FAD5, FAD7, and DGD1 genes. Since exogenous application of glycerol converts wild-type plants into ssi2 mimics, we also studied the effect of exogenous application of glycerol on mutants impaired in resistance-gene signaling, SA, or fad pathways. Glycerol increased SA levels and induced pathogenesis-related gene expression in all but sid2, nahG, fad7, and fad7 fad8 plants. Furthermore, glycerol-induced phenotypes in various mutant lines correlate with a concomitant reduction in 18:1 levels. Inability to convert glycerol into G3P due to a mutation in the nho1-encoded glycerol kinase renders plants tolerant to glycerol and unable to induce the SA-dependent pathway. A reduction in the NHO1-derived G3P pool also results in a partial age-dependent rescue of the ssi2 morphological and cell death phenotypes in the ssi2 nho1 plants. The glycerol-mediated induction of defense was not associated with any major changes in the lipid profile and/or levels of phosphatidic acid. Taken together, our results suggest that glycerol application and the ssi2 mutation in various mutant backgrounds produce similar effects and that restoration of ssi2 phenotypes is not associated with the further desaturation of 18:1 to linoleic or linolenic acids in plastidal or extraplastidal lipids.
Figures







References
-
- Browse J, McConn M, James D, Miquel M (1993) Mutants of Arabidopsis deficient in the synthesis of α-Linolenate: biochemical and genetic characterization of the endoplasmic reticulum linoleoyl desaturase. J Biol Chem 268: 16345–16351 - PubMed
-
- Browse J, McCourt P, Somerville CR (1985) A mutant of Arabidopsis lacking a chloroplast-specific lipid. Science 277: 763–765 - PubMed
-
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 - PubMed
-
- Century KS, Shapiro AD, Repetti PP, Dahlbeck D, Holub E, Staskawicz BJ (1997) NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278: 1963–1965 - PubMed
-
- Chandra-Shekara AC, Gupte M, Navarre DA, Raina S, Raina R, Klessig D, Kachroo P (2006) Light-dependent hypersensitive response and resistance signaling to turnip crinkle virus in Arabidopsis. Plant J (in press) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

