The adult mouse hippocampal progenitor is neurogenic but not a stem cell
- PMID: 16306394
- PMCID: PMC6725873
- DOI: 10.1523/JNEUROSCI.3249-05.2005
The adult mouse hippocampal progenitor is neurogenic but not a stem cell
Abstract
The aim of this investigation was to characterize the proliferative precursor cells in the adult mouse hippocampal region. Given that a very large number of new hippocampal cells are generated over the lifetime of an animal, it is predicted that a neural stem cell is ultimately responsible for maintaining this genesis. Although it is generally accepted that a proliferative precursor resides within the hippocampus, contradictory reports exist regarding the classification of this cell. Is it a true stem cell or a more limited progenitor? Using a strict functional definition of a neural stem cell and a number of in vitro assays, we report that the resident hippocampal precursor is a progenitor capable of proliferation and multipotential differentiation but is unable to self-renew and thus proliferate indefinitely. Furthermore, the mitogen FGF-2 stimulates proliferation of these cells to a greater extent than epidermal growth factor (EGF). In addition, we found that BDNF was essential for the production of neurons from the hippocampal progenitor cells, being required during proliferation to trigger neuronal fate. In contrast, a bona fide neural stem cell was identified in the lateral wall of the lateral ventricle surrounding the hippocampus. Interestingly, EGF proved to be the stronger mitogenic factor for this cell, which was clearly a different precursor from the resident hippocampal progenitor. These results suggest that the stem cell ultimately responsible for adult hippocampal neurogenesis resides outside the hippocampus, producing progenitor cells that migrate into the neurogenic zones and proliferate to produce new neurons and glia.
Figures

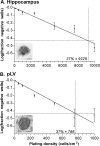




References
-
- Adams DJ, Reynolds BA, Louis SA, Rietze RL (2004) The neurosphere assay overestimates the frequency of adult neural stem cells. Soc Neurosci Abstr 30: 383.6.
-
- Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319-335. - PubMed
-
- Becq H, Jorquera I, Ben-Ari Y, Weiss S, Represa A (2005) Differential properties of dentate gyrus and CA1 neural precursors. J Neurobiol 62: 243-261. - PubMed
-
- Cameron HA, Woolley CS, McEwen BS, Gould E (1993) Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56: 337-344. - PubMed
-
- Cheng Y, Black IB, DiCicco-Bloom E (2002) Hippocampal granule neuron production and population size are regulated by levels of bFGF. Eur J Neurosci 15: 3-12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
