Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I
- PMID: 16306401
- PMCID: PMC6725866
- DOI: 10.1523/JNEUROSCI.2909-05.2005
Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I
Abstract
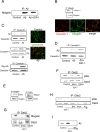
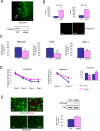
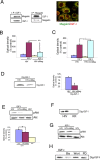
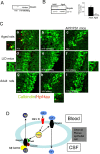
The involvement of circulating insulin-like growth factor I (IGF-I) in the beneficial effects of physical exercise on the brain makes this abundant serum growth factor a physiologically relevant neuroprotective signal. However, the mechanisms underlying neuroprotection by serum IGF-I remain primarily unknown. Among many other neuroprotective actions, IGF-I enhances clearance of brain amyloid beta (Abeta) by modulating transport/production of Abeta carriers at the blood-brain interface in the choroid plexus. We found that physical exercise increases the levels of the choroid plexus endocytic receptor megalin/low-density lipoprotein receptor-related protein-2 (LRP2), a multicargo transporter known to participate in brain uptake of Abeta carriers. By manipulating choroid plexus megalin levels through viral-directed overexpression and RNA interference, we observed that megalin mediates IGF-I-induced clearance of Abeta and is involved in IGF-I transport into the brain. Through this dual role, megalin participates in the neuroprotective actions of IGF-I including prevention of tau hyperphosphorylation and maintenance of cognitive function in a variety of animal models of cognitive loss. Because we found that in normal aged animals, choroid plexus megalin/LRP2 is decreased, an attenuated IGF-I/megalin input may contribute to increased risk of neurodegeneration, including late-onset Alzheimer's disease.
Figures


References
-
- Avila J, Lucas JJ, Perez M, Hernandez F (2004) Role of tau protein in both physiological and pathological conditions. Physiol Rev 84: 361-384. - PubMed
-
- Barth JL, Argraves WS (2001) Cubilin and megalin: partners in lipoprotein and vitamin metabolism. Trends Cardiovasc Med 11: 26-31. - PubMed
-
- Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ (1996) Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem 271: 32916-32922. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
