Neural activity controls the synaptic accumulation of alpha-synuclein
- PMID: 16306404
- PMCID: PMC6725870
- DOI: 10.1523/JNEUROSCI.2922-05.2005
Neural activity controls the synaptic accumulation of alpha-synuclein
Abstract
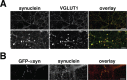
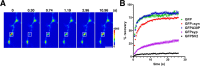
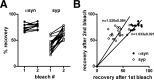
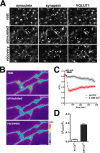
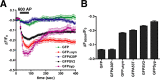
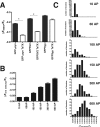
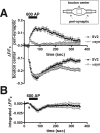
The presynaptic protein alpha-synuclein has a central role in Parkinson's disease (PD). However, the mechanism by which the protein contributes to neurodegeneration and its normal function remain unknown. Alpha-synuclein localizes to the nerve terminal and interacts with artificial membranes in vitro but binds weakly to native brain membranes. To characterize the membrane association of alpha-synuclein in living neurons, we used fluorescence recovery after photobleaching. Despite its enrichment at the synapse, alpha-synuclein is highly mobile, with rapid exchange between adjacent synapses. In addition, we find that alpha-synuclein disperses from the nerve terminal in response to neural activity. Dispersion depends on exocytosis, but unlike other synaptic vesicle proteins, alpha-synuclein dissociates from the synaptic vesicle membrane after fusion. Furthermore, the dispersion of alpha-synuclein is graded with respect to stimulus intensity. Neural activity thus controls the normal function of alpha-synuclein at the nerve terminal and may influence its role in PD.
Figures
Similar articles
-
The physiological role of α-synuclein and its relationship to Parkinson's Disease.J Neurochem. 2019 Sep;150(5):475-486. doi: 10.1111/jnc.14810. Epub 2019 Jul 28. J Neurochem. 2019. PMID: 31269263 Free PMC article. Review.
-
Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein.J Neurosci. 2002 Oct 15;22(20):8797-807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. J Neurosci. 2002. PMID: 12388586 Free PMC article.
-
Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis.J Neurosci. 2006 Nov 15;26(46):11915-22. doi: 10.1523/JNEUROSCI.3821-06.2006. J Neurosci. 2006. PMID: 17108165 Free PMC article.
-
Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis.Neuron. 2010 Jan 14;65(1):66-79. doi: 10.1016/j.neuron.2009.12.023. Neuron. 2010. PMID: 20152114 Free PMC article.
-
Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse?FASEB J. 2004 Apr;18(6):637-47. doi: 10.1096/fj.03-1112rev. FASEB J. 2004. PMID: 15054086 Review.
Cited by
-
Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein.Exp Neurol. 2012 Oct;237(2):318-34. doi: 10.1016/j.expneurol.2012.06.025. Epub 2012 Jun 27. Exp Neurol. 2012. PMID: 22750327 Free PMC article.
-
Are we listening to everything the PARK genes are telling us?J Comp Neurol. 2019 May 15;527(9):1527-1540. doi: 10.1002/cne.24642. Epub 2019 Feb 8. J Comp Neurol. 2019. PMID: 30680728 Free PMC article. Review.
-
The physiological role of α-synuclein and its relationship to Parkinson's Disease.J Neurochem. 2019 Sep;150(5):475-486. doi: 10.1111/jnc.14810. Epub 2019 Jul 28. J Neurochem. 2019. PMID: 31269263 Free PMC article. Review.
-
On the key role played by altered protein conformation in Parkinson's disease.J Neural Transm (Vienna). 2008 Sep;115(9):1285-99. doi: 10.1007/s00702-008-0072-1. Epub 2008 Jun 5. J Neural Transm (Vienna). 2008. PMID: 18528629 Review.
-
Traumatic brain injury dysregulates microRNAs to modulate cell signaling in rat hippocampus.PLoS One. 2014 Aug 4;9(8):e103948. doi: 10.1371/journal.pone.0103948. eCollection 2014. PLoS One. 2014. PMID: 25089700 Free PMC article.
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239-252. - PubMed
-
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci 22: 8797-8807. - PMC - PubMed
-
- Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC (2003) A broken alpha-helix in folded alpha-synuclein. J Biol Chem 278: 15313-15318. - PubMed
-
- Chi P, Greengard P, Ryan TA (2001) Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci 4: 1187-1193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
