Emerging patterns of neuronal responses in supplementary and primary motor areas during sensorimotor adaptation
- PMID: 16306407
- PMCID: PMC6725878
- DOI: 10.1523/JNEUROSCI.0164-05.2005
Emerging patterns of neuronal responses in supplementary and primary motor areas during sensorimotor adaptation
Abstract
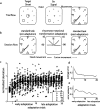
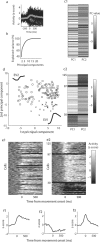
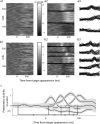
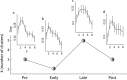
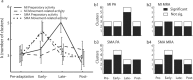
Acquisition and retention of sensorimotor skills have been extensively investigated psychophysically, but little is known about the underlying neuronal mechanisms. Here we examine the evolution of neural activity associated with adaptation to new kinematic tasks in two cortical areas: the caudal supplementary motor area (SMA proper), and the primary motor cortex (MI). We investigate the hypothesis that adaptation starts at premotor areas, i.e., higher in the hierarchy of computation, until a stable representation is formed in primary areas. In accordance with previous studies, we found that adaptation can be characterized by two phases: an early phase that is accompanied by fast and substantial reduction of errors, followed by a late phase with slower and more moderate improvements in behavior. We used unsupervised clustering to separate the activity of the single cells into groups of cells with similar response patterns, under the assumption that each such subpopulation forms a functional unit. We specifically observed the number of clusters in each cortical area during early and late phases of the adaptation and found that the number of clusters is higher in the SMA during early phases of adaptation. In contrast, a higher number of clusters was observed in MI only during late phases. Our results suggest a new approach to analyze responses of large populations of neurons and use it to show a hierarchy of dynamic reorganization of functional groups during adaptation.
Figures




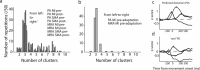
References
-
- Aizawa H, Inase M, Mushiake H, Shima K, Tanji J (1991) Reorganization of activity in the supplementary motor area associated with motor learning and functional recovery. Exp Brain Res 84: 668-671. - PubMed
-
- Alexander GE, Crutcher MD (1990) Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol 64: 133-150. - PubMed
-
- Brasted PJ, Wise SP (2004) Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci 19: 721-740. - PubMed
-
- Cisek P, Scott SH (1999) An alternative interpretation of population vector rotation in macaque motor cortex. Neurosci Lett 272: 1-4. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
