Upregulation of the voltage-gated sodium channel beta2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons
- PMID: 16306410
- PMCID: PMC6725885
- DOI: 10.1523/JNEUROSCI.3066-05.2005
Upregulation of the voltage-gated sodium channel beta2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons
Abstract
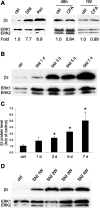
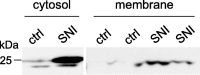
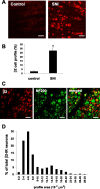
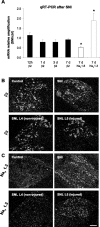
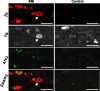
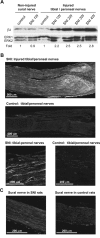
The development of abnormal primary sensory neuron excitability and neuropathic pain symptoms after peripheral nerve injury is associated with altered expression of voltage-gated sodium channels (VGSCs) and a modification of sodium currents. To investigate whether the beta2 subunit of VGSCs participates in the generation of neuropathic pain, we used the spared nerve injury (SNI) model in rats to examine beta2 subunit expression in selectively injured (tibial and common peroneal nerves) and uninjured (sural nerve) afferents. Three days after SNI, immunohistochemistry and Western blot analysis reveal an increase in the beta2 subunit in both the cell body and peripheral axons of injured neurons. The increase persists for >4 weeks, although beta2 subunit mRNA measured by real-time reverse transcription-PCR and in situ hybridization remains unchanged. Although injured neurons show the most marked upregulation,beta2 subunit expression is also increased in neighboring non-injured neurons and a similar pattern of changes appears in the spinal nerve ligation model of neuropathic pain. That increased beta2 subunit expression in sensory neurons after nerve injury is functionally significant, as demonstrated by our finding that the development of mechanical allodynia-like behavior in the SNI model is attenuated in beta2 subunit null mutant mice. Through its role in regulating the density of mature VGSC complexes in the plasma membrane and modulating channel gating, the beta2 subunit may play a key role in the development of ectopic activity in injured and non-injured sensory afferents and, thereby, neuropathic pain.
Figures



Similar articles
-
Expression of auxiliary beta subunits of sodium channels in primary afferent neurons and the effect of nerve injury.Neuroscience. 2003;121(2):441-50. doi: 10.1016/s0306-4522(03)00432-9. Neuroscience. 2003. PMID: 14522002
-
The pattern of expression of the voltage-gated sodium channels Na(v)1.8 and Na(v)1.9 does not change in uninjured primary sensory neurons in experimental neuropathic pain models.Pain. 2002 Apr;96(3):269-277. doi: 10.1016/S0304-3959(01)00456-0. Pain. 2002. PMID: 11972999
-
Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats.Pain. 2005 Sep;117(1-2):145-53. doi: 10.1016/j.pain.2005.05.027. Pain. 2005. PMID: 16061326
-
Neuronal voltage-gated sodium channel subtypes: key roles in inflammatory and neuropathic pain.Int J Biochem Cell Biol. 2006;38(12):2005-10. doi: 10.1016/j.biocel.2006.06.008. Epub 2006 Jul 12. Int J Biochem Cell Biol. 2006. PMID: 16919992 Review.
-
Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions.J Neurosci. 1998 Mar 15;18(6):2174-87. doi: 10.1523/JNEUROSCI.18-06-02174.1998. J Neurosci. 1998. PMID: 9482802 Free PMC article. Review.
Cited by
-
MicroRNA‑449a regulates the progression of brain aging by targeting SCN2B in SAMP8 mice.Int J Mol Med. 2020 Apr;45(4):1091-1102. doi: 10.3892/ijmm.2020.4502. Epub 2020 Feb 13. Int J Mol Med. 2020. PMID: 32124967 Free PMC article.
-
SCN2B in the Rat Trigeminal Ganglion and Trigeminal Sensory Nuclei.Cell Mol Neurobiol. 2016 Nov;36(8):1399-1408. doi: 10.1007/s10571-016-0340-9. Epub 2016 Feb 6. Cell Mol Neurobiol. 2016. PMID: 26852328 Free PMC article.
-
Alzheimer's secretases regulate voltage-gated sodium channels.Neurosci Lett. 2010 Dec 10;486(2):68-72. doi: 10.1016/j.neulet.2010.08.048. Epub 2010 Sep 15. Neurosci Lett. 2010. PMID: 20817076 Free PMC article. Review.
-
Na Channel β Subunits: Overachievers of the Ion Channel Family.Front Pharmacol. 2011 Sep 28;2:53. doi: 10.3389/fphar.2011.00053. eCollection 2011. Front Pharmacol. 2011. PMID: 22007171 Free PMC article.
-
Multivariate gene expression analysis reveals functional connectivity changes between normal/tumoral prostates.BMC Syst Biol. 2008 Dec 5;2:106. doi: 10.1186/1752-0509-2-106. BMC Syst Biol. 2008. PMID: 19055846 Free PMC article.
References
-
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ (2000) Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci 15: 331-342. - PubMed
-
- Amir R, Devor M (2000) Functional cross-excitation between afferent A- and C-neurons in dorsal root ganglia. Neuroscience 95: 189-195. - PubMed
-
- Benn SC, Perrelet D, Kato A, Scholz J, Decosterd I, Mannion R, Bakowska J, Woolf C (2002) Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron 36: 45-56. - PubMed
-
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG (1996) Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain Res Mol Brain Res 43: 117-131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
