Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states
- PMID: 16306412
- PMCID: PMC6725889
- DOI: 10.1523/JNEUROSCI.3229-05.2005
Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states
Abstract
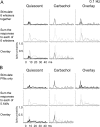
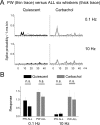
The main role of the thalamus is to relay sensory inputs to the neocortex according to the regulations dictated by behavioral state. Hence, changes in behavioral state are likely to transform the temporal and spatial properties of thalamocortical receptive fields. We compared the receptive fields of single cells in the ventroposterior medial thalamus (VPM) of urethane-anesthetized rats during quiescent states and during aroused (activated) states. During quiescent states, VPM cells respond to stimulation of a principal whisker (PW) and may respond modestly to one or a few adjacent whiskers (AWs). During either generalized forebrain activation or selective thalamic activation caused by carbachol infusion in the VPM, the responses to AWs enhance so that VPM receptive fields become much larger. Such enlargement is not observed at the level of the principal trigeminal nucleus, indicating that it originates within the thalamus. Interestingly, despite the increase in AW responses during activation, simultaneous deflection of the PW and AWs produced VPM responses that resembled the PW response, as if the AWs were not stimulated. This nonlinear summation of sensory responses was present during both quiescent and activated states. In conclusion, the thalamus suppresses the excitatory surround (AWs) of the receptive field during quiescent states and enlarges this surround during arousal. But, thalamocortical cells represent only the center (PW) of the receptive field when the center (PW) and surround (AWs) are stimulated simultaneously.
Figures





References
-
- Ahissar E, Sosnik R, Haidarliu S (2000) Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406: 302-306. - PubMed
-
- Armstrong-James M, Callahan CA (1991) Thalamo-cortical processing of vibrissal information in the rat. II. spatiotemporal convergence in the thalamic ventroposterior medial nucleus (VPm) and its relevance to generation of receptive fields of S1 cortical “barrel” neurones. J Comp Neurol 303: 211-224. - PubMed
-
- Berg RW, Friedman B, Schroeder LF, Kleinfeld D (2005) Activation of nucleus basalis facilitates cortical control of a brain stem motor program 2. J Neurophysiol 94: 699-711. - PubMed
-
- Bernardo KL, Woolsey TA (1987) Axonal trajectories between mouse somatosensory thalamus and cortex. J Comp Neurol 258: 542-564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
