Prediction and decoding of retinal ganglion cell responses with a probabilistic spiking model
- PMID: 16306413
- PMCID: PMC6725882
- DOI: 10.1523/JNEUROSCI.3305-05.2005
Prediction and decoding of retinal ganglion cell responses with a probabilistic spiking model
Abstract
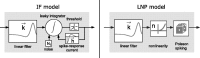
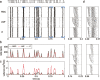
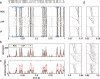
Sensory encoding in spiking neurons depends on both the integration of sensory inputs and the intrinsic dynamics and variability of spike generation. We show that the stimulus selectivity, reliability, and timing precision of primate retinal ganglion cell (RGC) light responses can be reproduced accurately with a simple model consisting of a leaky integrate-and-fire spike generator driven by a linearly filtered stimulus, a postspike current, and a Gaussian noise current. We fit model parameters for individual RGCs by maximizing the likelihood of observed spike responses to a stochastic visual stimulus. Although compact, the fitted model predicts the detailed time structure of responses to novel stimuli, accurately capturing the interaction between the spiking history and sensory stimulus selectivity. The model also accounts for the variability in responses to repeated stimuli, even when fit to data from a single (nonrepeating) stimulus sequence. Finally, the model can be used to derive an explicit, maximum-likelihood decoding rule for neural spike trains, thus providing a tool for assessing the limitations that spiking variability imposes on sensory performance.
Figures






Similar articles
-
Decoding visual information from a population of retinal ganglion cells.J Neurophysiol. 1997 Nov;78(5):2336-50. doi: 10.1152/jn.1997.78.5.2336. J Neurophysiol. 1997. PMID: 9356386
-
The power ratio and the interval map: spiking models and extracellular recordings.J Neurosci. 1998 Dec 1;18(23):10090-104. doi: 10.1523/JNEUROSCI.18-23-10090.1998. J Neurosci. 1998. PMID: 9822763 Free PMC article.
-
Modeling the impact of common noise inputs on the network activity of retinal ganglion cells.J Comput Neurosci. 2012 Aug;33(1):97-121. doi: 10.1007/s10827-011-0376-2. Epub 2011 Dec 29. J Comput Neurosci. 2012. PMID: 22203465 Free PMC article.
-
Statistical models for neural encoding, decoding, and optimal stimulus design.Prog Brain Res. 2007;165:493-507. doi: 10.1016/S0079-6123(06)65031-0. Prog Brain Res. 2007. PMID: 17925266 Review.
-
Refractoriness and neural precision.J Neurosci. 1998 Mar 15;18(6):2200-11. doi: 10.1523/JNEUROSCI.18-06-02200.1998. J Neurosci. 1998. PMID: 9482804 Free PMC article. Review.
Cited by
-
Identifying and tracking simulated synaptic inputs from neuronal firing: insights from in vitro experiments.PLoS Comput Biol. 2015 Mar 30;11(3):e1004167. doi: 10.1371/journal.pcbi.1004167. eCollection 2015 Mar. PLoS Comput Biol. 2015. PMID: 25823000 Free PMC article.
-
Multiple spike time patterns occur at bifurcation points of membrane potential dynamics.PLoS Comput Biol. 2012;8(10):e1002615. doi: 10.1371/journal.pcbi.1002615. Epub 2012 Oct 18. PLoS Comput Biol. 2012. PMID: 23093916 Free PMC article.
-
The accuracy of membrane potential reconstruction based on spiking receptive fields.J Neurophysiol. 2012 Apr;107(8):2143-53. doi: 10.1152/jn.01176.2011. Epub 2012 Jan 25. J Neurophysiol. 2012. PMID: 22279194 Free PMC article.
-
Neural coding and perception of auditory motion direction based on interaural time differences.J Neurophysiol. 2019 Oct 1;122(4):1821-1842. doi: 10.1152/jn.00081.2019. Epub 2019 Aug 28. J Neurophysiol. 2019. PMID: 31461376 Free PMC article.
-
Subpopulations of neurons in lOFC encode previous and current rewards at time of choice.Elife. 2021 Oct 25;10:e70129. doi: 10.7554/eLife.70129. Elife. 2021. PMID: 34693908 Free PMC article.
References
-
- Aguera y Arcas B, Fairhall AL (2003) What causes a neuron to spike? Neural Comput 15: 1789-1807. - PubMed
-
- Baccus SA, Meister M (2002) Fast and slow contrast adaptation in retinal circuitry. Neuron 36: 909-919. - PubMed
-
- Banerjee A (2001) On the phase-space dynamics of systems of spiking neurons, ii: formal analysis. Neural Comput 13: 195-225. - PubMed
-
- Benardete EA, Kaplan E, Knight BW (1992) Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Vis Neurosci 8: 483-486. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
