Cardiomyocyte cell cycle activation ameliorates fibrosis in the atrium
- PMID: 16306446
- PMCID: PMC2696639
- DOI: 10.1161/01.RES.0000197783.70106.4a
Cardiomyocyte cell cycle activation ameliorates fibrosis in the atrium
Abstract
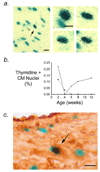
MHC-TGFcys33ser transgenic mice have elevated levels of active transforming growth factor (TGF)-beta1 in the myocardium. Previous studies have shown that these animals develop atrial, but not ventricular, fibrosis. Here we show that atrial fibrosis was accompanied with cardiomyocyte apoptosis. Although similar levels of cardiomyocyte apoptosis were present in the right and left atria of MHC-TGFcys33ser hearts, the extent of fibrosis was more pronounced in the right atrium. Thus, additional factors influence the degree of atrial fibrosis in this model. Tritiated thymidine incorporation studies revealed cardiomyocyte cell cycle activity in left atrial cardiomyocytes, but not in right atrial cardiomyocytes. These observations suggested that cardiomyocyte cell cycle activation ameliorated the severity of atrial fibrosis. To directly test this hypothesis, MHC-TGFcys33ser mice were crossed with MHC-cycD2 mice (which have constitutive cardiomyocyte cell cycle activity in the right atrium). Mice inheriting both transgenes exhibited right atrial cardiomyocyte cell cycle activity and a concomitant reduction in the severity of right atrial fibrosis, despite the presence of a similar level of cardiomyocyte apoptosis as was observed in mice inheriting the MHC-TGFcys33ser transgene alone. These data support the notion that cardiomyocyte cell cycle induction can antagonize fibrosis in the myocardium.
Figures



References
-
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. - PubMed
-
- Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. - PubMed
-
- Butt RP, Laurent GJ, Bishop JE. Collagen production and replication by cardiac fibroblasts is enhanced in response to diverse classes of growth factors. Eur J Cell Biol. 1995;68:330–335. - PubMed
-
- Boluyt MO, O'Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH, Crow MT, Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res. 1994;75:23–32. - PubMed
-
- Butt RP, Laurent GJ, Bishop JE. Mechanical load and polypeptide growth factors stimulate cardiac fibroblast activity. Ann N Y Acad Sci. 1995;752:387–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

