Novel caprine adeno-associated virus (AAV) capsid (AAV-Go.1) is closely related to the primate AAV-5 and has unique tropism and neutralization properties
- PMID: 16306595
- PMCID: PMC1316006
- DOI: 10.1128/JVI.79.24.15238-15245.2005
Novel caprine adeno-associated virus (AAV) capsid (AAV-Go.1) is closely related to the primate AAV-5 and has unique tropism and neutralization properties
Abstract
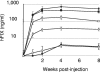
Preexisting humoral immunity to adeno-associated virus (AAV) vectors may limit their clinical utility in gene delivery. We describe a novel caprine AAV (AAV-Go.1) capsid with unique biological properties. AAV-Go.1 capsid was cloned from goat-derived adenovirus preparations. Surprisingly, AAV-Go.1 capsid was 94% identical to the human AAV-5, with differences predicted to be largely on the surface and on or under the spike-like protrusions. In an in vitro neutralization assay using human immunoglobulin G (IgG) (intravenous immune globulin [IVIG]), AAV-Go.1 had higher resistance than AAV-5 (100-fold) and resistance similar to that of AAV-4 or AAV-8. In an in vivo model, SCID mice were pretreated with IVIG to generate normal human IgG plasma levels prior to the administration of AAV human factor IX vectors. Protein expression after intramuscular administration of AAV-Go.1 was unaffected in IVIG-pretreated mice, while it was reduced 5- and 10-fold after administration of AAV-1 and AAV-8, respectively. In contrast, protein expression after intravenous administration of AAV-Go.1 was reduced 7.1-fold, similar to the 3.8-fold reduction observed after AAV-8 administration in IVIG-pretreated mice, and protein expression was essentially extinguished after AAV-2 administration in mice pretreated with much less IVIG (15-fold). AAV-Go.1 vectors also demonstrated a marked tropism for lung when administered intravenously in SCID mice. The pulmonary tropism and high neutralization resistance to human preexisting antibodies suggest novel therapeutic uses for AAV-Go.1 vectors, including targeting diseases such as cystic fibrosis. Nonprimate sources of AAVs may be useful to identify additional capsids with distinct tropisms and high resistance to neutralization by human preexisting antibodies.
Figures

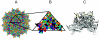

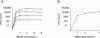
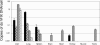
References
-
- Bankiewicz, K. S., J. L. Eberling, M. Kohutnicka, W. Jagust, P. Pivirotto, J. Bringas, J. Cunningham, T. F. Budinger, and J. Harvey-White. 2000. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 164:2-14. - PubMed
-
- Chao, H., Y. Liu, J. Rabinowitz, C. Li, R. J. Samulski, and C. E. Walsh. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2:619-623. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

