Respiratory syncytial virus-inducible BCL-3 expression antagonizes the STAT/IRF and NF-kappaB signaling pathways by inducing histone deacetylase 1 recruitment to the interleukin-8 promoter
- PMID: 16306601
- PMCID: PMC1316019
- DOI: 10.1128/JVI.79.24.15302-15313.2005
Respiratory syncytial virus-inducible BCL-3 expression antagonizes the STAT/IRF and NF-kappaB signaling pathways by inducing histone deacetylase 1 recruitment to the interleukin-8 promoter
Abstract
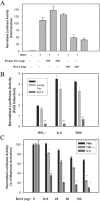
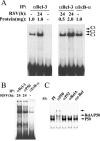
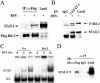
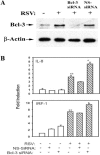
Respiratory syncytial virus (RSV) is a paramyxovirus that produces airway inflammation, in part by inducing interleukin-8 (IL-8) expression, a CXC-type chemokine, via the NF-kappaB/RelA and STAT/IRF signaling pathways. In RSV-infected A549 cells, IL-8 transcription attenuates after 24 h in spite of ongoing viral replication and persistence of nuclear RelA, suggesting a mechanism for transcriptional attenuation. RSV infection induces B-cell lymphoma protein -3 (Bcl-3) expression 6 to 12 h after viral infection, at times when IL-8 transcription is inhibited. By contrast, 293 cells, deficient in inducible Bcl-3 expression, show no attenuation of IL-8 transcription. We therefore examined Bcl-3's role in terminating virus-inducible IL-8 transcription. Transient expression of Bcl-3 potently inhibited virus-inducible IL-8 transcription by disrupting both the NF-kappaB and STAT/IRF pathways. Although previously Bcl-3 was thought to capture 50-kDa NF-kappaB1 isoforms in the cytoplasm, immunoprecipitation (IP) and electrophoretic mobility shift assays indicate that nuclear Bcl-3 associates with NF-kappaB1 without affecting DNA binding. Additionally, Bcl-3 potently inhibited the STAT/IRF pathway. Nondenaturing co-IP assays indicate that nuclear Bcl-3 associates with STAT-1 and histone deacetylase 1 (HDAC-1), increasing HDAC-1 recruitment to the IL-8 promoter. Treatment with the HDAC inhibitor trichostatin A blocks attenuation of IL-8 transcription. A nuclear targeting-deficient Bcl-3 is unable to enhance HDAC-1-mediated chemokine repression. Finally, small inhibitory RNA-mediated Bcl-3 "knockdown" resulted in enhanced RSV-induced chemokine expression in A549 cells. These data indicate that Bcl-3 is a virus-inducible inhibitor of chemokine transcription by interfering with the NF-kappaB and STAT/IRF signaling pathways by complexing with them and recruiting HDAC-1 to attenuate target promoter activity.
Figures



Similar articles
-
Respiratory syncytial virus influences NF-kappaB-dependent gene expression through a novel pathway involving MAP3K14/NIK expression and nuclear complex formation with NF-kappaB2.J Virol. 2005 Jul;79(14):8948-59. doi: 10.1128/JVI.79.14.8948-8959.2005. J Virol. 2005. PMID: 15994789 Free PMC article.
-
Respiratory syncytial virus and TNF alpha induction of chemokine gene expression involves differential activation of Rel A and NF-kappa B1.BMC Infect Dis. 2002 Mar 28;2:5. doi: 10.1186/1471-2334-2-5. BMC Infect Dis. 2002. PMID: 11922866 Free PMC article.
-
Histone deacetylase inhibitors suppress RSV infection and alleviate virus-induced airway inflammation.Int J Mol Med. 2016 Sep;38(3):812-22. doi: 10.3892/ijmm.2016.2691. Epub 2016 Jul 26. Int J Mol Med. 2016. PMID: 27460781 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-kappaB.J Leukoc Biol. 1999 Mar;65(3):291-8. doi: 10.1002/jlb.65.3.291. J Leukoc Biol. 1999. PMID: 10080530 Review.
Cited by
-
Regulation of Retinoic Acid Inducible Gene-I (RIG-I) Activation by the Histone Deacetylase 6.EBioMedicine. 2016 Jul;9:195-206. doi: 10.1016/j.ebiom.2016.06.015. Epub 2016 Jun 11. EBioMedicine. 2016. PMID: 27372014 Free PMC article.
-
BCL-3 loss sensitises colorectal cancer cells to DNA damage by targeting homologous recombination.DNA Repair (Amst). 2022 Jul;115:103331. doi: 10.1016/j.dnarep.2022.103331. Epub 2022 Apr 16. DNA Repair (Amst). 2022. PMID: 35468497 Free PMC article.
-
Combination Therapies Targeting HDAC and IKK in Solid Tumors.Trends Pharmacol Sci. 2018 Mar;39(3):295-306. doi: 10.1016/j.tips.2017.11.008. Epub 2017 Dec 9. Trends Pharmacol Sci. 2018. PMID: 29233541 Free PMC article. Review.
-
BCL-3 attenuation of TNFA expression involves an incoherent feed-forward loop regulated by chromatin structure.PLoS One. 2013 Oct 10;8(10):e77015. doi: 10.1371/journal.pone.0077015. eCollection 2013. PLoS One. 2013. PMID: 24130828 Free PMC article.
-
Establishment of a porcine bronchial epithelial cell line and its application to study innate immunity in the respiratory epithelium.Front Immunol. 2023 Jul 3;14:1117102. doi: 10.3389/fimmu.2023.1117102. eCollection 2023. Front Immunol. 2023. PMID: 37465671 Free PMC article.
References
-
- Alexander, W. S., R. Starr, J. E. Fenner, C. L. Scott, E. Handman, N. S. Sprigg, J. E. Corbin, A. L. Cornish, R. Darwiche, C. M. Owczarek, T. W. Kay, N. A. Nicola, P. J. Hertzog, D. Metcalf, and D. J. Hilton. 1999. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98:597-608. - PubMed
-
- Baggiolini, M., B. Dewald, and B. Moser. 1997. Human chemokines: an update. Annu. Rev. Immunol. 15:675-705. - PubMed
-
- Barnes, P. J., and M. Karin. 1997. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066-1071. - PubMed
-
- Bitko, V., A. Velazquez, L. Yank, Y.-C. Yang, and S. Barik. 1997. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-κB and is inhibited by sodium salicylate and aspirin. Virology 232:369-378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

