Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag
- PMID: 16306625
- PMCID: PMC1315991
- DOI: 10.1128/JVI.79.24.15547-15555.2005
Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag
Abstract
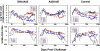
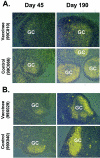
The prophylactic efficacy of DNA and replication-incompetent adenovirus serotype 5 (Ad5) vaccine vectors expressing simian immunodeficiency virus (SIV) Gag was examined in rhesus macaques using an SIVmac239 challenge. Cohorts of either Mamu-A*01(+) or Mamu-A*01(-) macaques were immunized with a DNA prime-Ad5 boost regimen; for comparison, a third cohort consisting of Mamu-A*01(+) monkeys was immunized using the Ad5 vector alone for both prime and boost. All animals, along with unvaccinated control cohorts of Mamu-A*01(+) and Mamu-A*01(-) macaques, were challenged intrarectally with SIVmac239. Viral loads were measured in both peripheral and lymphoid compartments. Only the DNA prime-Ad5-boosted Mamu-A*01(+) cohort exhibited a notable reduction in peak plasma viral load (sevenfold) as well as in early set-point viral burdens in both plasma and lymphoid tissues (10-fold) relative to those observed in the control monkeys sharing the same Mamu-A*01 allele. The degree of control in each animal correlated with the levels of Gag-specific immunity before virus challenge. However, virus control was short-lived, and indications of viral escape were evident as early as 6 months postinfection. The implications of these results in vaccine design and clinical testing are discussed.
Figures




References
-
- Allen, T. M., L. Mortara, B. R. Mothe, M. Liebl, P. C. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. X. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. - PMC - PubMed
-
- Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. - PMC - PubMed
-
- Barouch, D., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. - PMC - PubMed
-
- Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. - PubMed
-
- Caley, I. J., M. R. Betts, D. M. Irlbeck, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1997. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J. Virol. 71:3031-3038. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

